Nuclear processes - A Level Standard Questions
Q1.
Natural uranium consists of 99.3%  and 0.7%
and 0.7%  . In many nuclear reactors, the fuel consists of enriched uranium enclosed in sealed metal containers.
. In many nuclear reactors, the fuel consists of enriched uranium enclosed in sealed metal containers.
(a)
(i) Explain what is meant by enriched uranium.
(ii) Why is enriched uranium rather than natural uranium used in many nuclear reactors?
(2)
(b)
(i) By considering the neutrons involved in the fission process, explain how the rate of production of heat in a nuclear reactor is controlled.
(ii) Explain why all the fuel in a nuclear reactor is not placed in a single fuel rod.
(5)
(Total 7 marks)

Q2.
(a)
(i) Explain what is meant by the term binding energy for a nucleus.
(ii) Sketch a graph of the average binding energy per nucleon against nucleon number A, giving approximate values of the scale on each axis.
(b) Use your graph to explain why energy is released when a neutron collides with a U nucleus causing fission.
(2)
(c) Neutrons are released when nuclear fission occurs in U. Some of these neutrons induce further fission, others are absorbed without further fission and others escape from the surface of the material. The average number of neutrons released per fission is 2.5, of which at least one must produce further fission if a chain reaction is to be sustained.
Explain how a chain reaction can occur only if the piece of uranium has a certain minimum mass (the critical mass).
(3)
(Total 10 marks)

Q3.
(a) Explain why, after a period of use, the fuel rods in a nuclear reactor become:
(i) less effective for power production,
(ii) more dangerous.
(3)
(b) Describe the stages in the handling and processing of spent fuel rods after they have been removed from a reactor, indicating how the active wastes are dealt with.
(5)
(Total 8 marks)

Q4.
(a) The unstable uranium nucleus 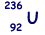 is produced in a nuclear reactor.
is produced in a nuclear reactor.
(i) Copy and complete the equation which shows the formation of 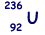
+ 
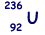
(ii) 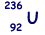 can decay by nuclear fission in many different ways.
can decay by nuclear fission in many different ways.
Complete the equation which shows one possible decay channel.

(2 marks)
(b) Calculate the energy released, in MeV, in the fission reaction.
mass of a neutron = 1.00867u
atomic mass of Ba = 144.92694u
atomic mass of U = 236.04573u
atomic mass of = 86.91340u
(3 marks)
(Total 5 marks)

Q5.
(a) When 235U undergoes fission, the average number of neutrons produced per fission is about 2.5. Describe what happens to these neutrons in a thermal reactor which is producing power at a constant rate.
(5 marks)
(b) Write brief notes on the safety aspects of the following in the use of nuclear reactors for power production.
(i) use of control rods in normal operation and in emergency shut down
(ii) shielding
(iii) treatment of spent fuel rods
(8 marks)
(c) Explain how artificial isotopes are produced
(2 marks)
(Total 15 marks)

Q6. The moderator in a nuclear reactor is sometimes made of graphite. What is the purpose of the graphite?
A to absorb all the heat produced
B to decrease the neutron speeds
C to absorb the α and γ radiations
D to prevent the reactor from going critical







