Quantum Phenomena - discrete energy levels for electrons
Q5. The diagram below shows the lowest three energy levels of a hydrogen atom.
(a) An electron is incident on a hydrogen atom. As a result an electron in the ground state of the hydrogen atom is excited to the n = 2 energy level. The atom then emits a photon of a characteristic frequency.
(i) Explain why the electron in the ground state becomes excited to the n = 2 energy level.
The orbital electron needs to gain 13.6 - 3.41 eV of energy to make the transistion. It can make it if it absorbs enough energy from the incident electron when they interact.
It can make it if it absorbs enough energy from the incident electron when they interact.
OR
The incident electron loses energy to orbital electron when then interact.  If it transfers / 10.19eV, that is the required eergy needed to move up to level 2.
If it transfers / 10.19eV, that is the required eergy needed to move up to level 2.  Only that energy would be taken by the orbital electron (it cannot have KE left over!)
Only that energy would be taken by the orbital electron (it cannot have KE left over!)
(2 marks)
(ii) Calculate the frequency of the photon.
ΔE = (E2 –E1) = hf
ΔE = −3.41 − (− 13.6) = 10.19 eV 
energy of photon in joules = 10.19 × 1.6 × 10−19 = 1.63 × 10−18 J
6.63 × 10−34 × f = 1.63 × 10−18
f = 2.46 × 1015 Hz 
(3 marks)
(iii) The initial kinetic energy of the incident electron is 1.70 × 10−18 J. Calculate its kinetic energy after the collision.
Ek = 1.7 × 10−18 − 1.63 × 10−18 
Ek = 7.0 × 10−20 J 
(2 marks)
(iv) Show that the incident electron cannot excite the electron in the ground state to the n = 3 energy level.
The energy required to make the transition is 12.09 eV (or 1.9 × 10−18J ).
The energy of incident electron is only 10.63 eV (or 1.7 × 10−18 J) so it is of too low an energy for the transfer of energy required. 
(2 marks)
(b) When electrons in the ground state of hydrogen atoms are excited to the n = 3 energy level, photons of more than one frequency are subsequently released.
(i) Explain why different frequencies are possible.
Electrons return to lower levels by different routes. They may make one transition but they may go via intermediate energy stated, cascading to the lower energy state emiting photons from each stopping point along the way. 
(1 mark)
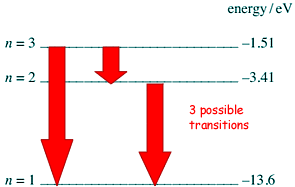
(ii) State and explain how many possible frequencies could be produced.
3 frequencies can be produced. 
These come from transitions from:
 n=3 to n=1
n=3 to n=1
 n=3 to n=2 and
n=3 to n=2 and
 n=2 to n=1
n=2 to n=1 
(2 marks)
(Total 12 marks)


