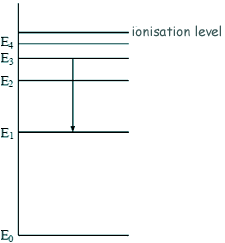
Energy Transistions - Multiple Choice Q1.The diagram shows some energy levels of an atom.
The transition E3 to E1 corresponds to the emission of visible light. A transition corresponding to the emission of infrared radiation could be:
So we are looking for a smaller transition - a transition to E2 (choice C or D) The photon is emitted not absorbed so the transition is to a lower energy number (choice D).
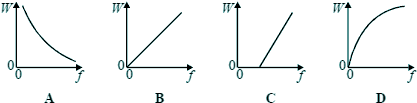
Q2. Which one of the graphs below best represents the relationship between the energy W of a photon and the frequency f of the radiation?
E=hf or in this case W = hf the equation of a straightline with c=0 (no intercept) Choice B
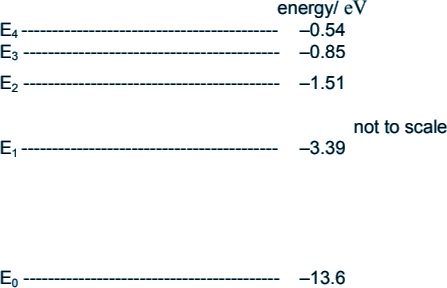
Q3. The diagram shows some of the energy levels for a hydrogen atom.
A free electron of kinetic energy 20.0 × 10–19 J collides with a hydrogen atom in its ground state. The hydrogen atom is excited from its ground state to the first excited state. Calculate the kinetic energy of the free electron after the collision in joules.
It uses (21.8 - 5.4) × 10–19 J of it to make the jump - 16.4 × 10–19 J. That leaves (20.0 - 16.4) × 10–19= 3.6 × 10–19J - choice B
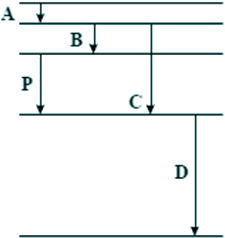
Q4. The diagram below is drawn to scale. It shows some of the energy levels of an atom.
Transition P results in the emission of a photon of wavelength 4 × 10–7 m. Which one of the transitions A, B, C, or D could result in the emission of a photon of wavelength 8 × 10–7 m?
Therefore the doubling of wavelength means halving the energy. Line B is half the length of line P - Choice B is the answer.
Q5. The diagram gives some of the energy levels of a hydrogen atom.
The transition of an excited hydrogen atom from E3 to E1 causes a photon of visible light to be emitted. Which transition causes a photon of ultraviolet light to be emitted?
-0.85 -(-3.39) = 2.54 eV
E4 to E3 = 0.31 eV E3 to E2 = 0.66 eV E2 to E1 = 1.88 eV Therefore the only possible transition is choice D - 10.21 eV
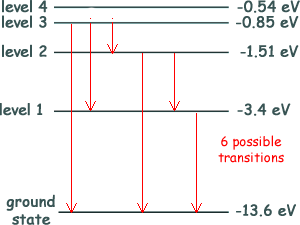
Q6. The diagram below shows an energy level diagram for a hydrogen atom.
Electrons with energy 13.0 eV collide with atoms of hydrogen in their ground state. What is the number of different wavelengths of electromagnetic radiation that could be emitted when the atoms de-excite? 13.0 eV is not the enough energy to ionise the atom but when it collides with a valency electron it can pass on part of its enegy and cause a promotion to all but level 4 (for level 4 promotion the valency electron would need 13.06 eV. Therefore the electron could promote to level 3. See the diagram to see the possible transitions from there... six possible (Choice C)
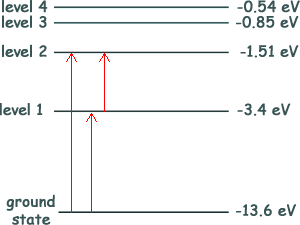
Q7. The diagram shows an energy-level diagram for a hydrogen atom.
Electrons, each having a kinetic energy of 2.0 × 10−18 J, collide with atoms of hydrogen in their ground state. Photons are emitted when the atoms de-excite. How many different wavelengths can be observed with incident electrons of this energy?
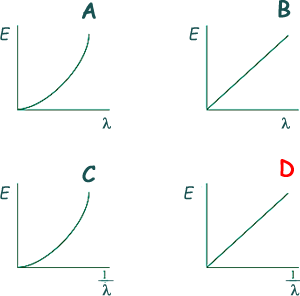
1 eV = 1.6 x 10-19 J 2.0 × 10−18 J = 2.0 × 10−18 /(1.6 x 10-19) eV 2.0 × 10−18 J = 12.5 eV - 13.6 + 12.5 = - 1.1 eV Therefore there would only be enough energy to promote electrons to level 2 - see diagram Choice B Q8. Which graph best shows the relationship between photon energy E and wavelength of a photon of electromagnetic radiation?
E = hf = hc/λ so E is directly proportional to 1/λ Choice D
|
Follow me...
|