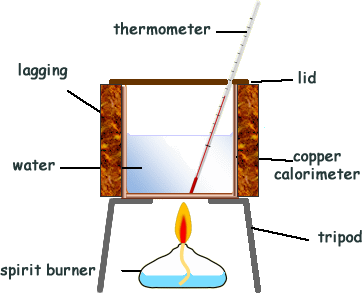
Calorimetry Measuring energy transfersEnergy can be released in chemical reactions as light, sound or electrical energy. But it is most often released as heat energy. This allows us to easily measure the amount of energy transferred. CalorimetryMeasuring heat transfers is called calorimetry. The diagram on the right shows a very simple calorimetry experiment to measure the heat energy released from burning fuel.
In a typical calorimetry experiment: The spirit burner containing the fuel is usually weighed before and after the experiment. In this way, the mass of the fuel burned can be found. Heat losses and/or gains are reduced as much as possible by using a lid, having the calorimenter as shiny as possible and lagging for the equipment. Fair testingWhen comparing different fuels, it is important to carry out a fair test. All variables should be kept constant, including:
More reliable results can be obtained by repeating the experiment many times. The biggest source of error in calorimetry is usually unwanted heat loss to the surroundings. This can be reduced by insulating the sides of the calorimeter and adding a lid. Calculating energy transfersThe amount of energy transferred from the burning fuel to the water in the calorimeter can be calculated if you know: This is the equation you need: energy transferred (joules, J) = mass of water heated (kg) × SHC of water × temperature rise (ºC) For a given amount of water heated up, the greater the temperature rise, the greater the amount of heat energy transferred to the water. For example, twice as much energy is transferred to the water to achieve a temperature increase of 20ºC compared with 10ºC. Comparing fuelsYou can compare fuels by measuring the mass of fuel burned in the experiment. The best fuel is likely to release the most energy per gram of fuel. This is worked out using: energy released (J/g of fuel) = energy transferred to water (J) ÷ mass of fuel burned (g)
When a fuel is burned a chemical reaction occurs:
This results in energy exchanges.
Whether a reaction is endothermic or exothermic overall depends on the difference between the energy needed to break bonds and the energy released when new bonds form. Calculating energy transferredWhat is the energy transferred to 100cm3 of water to raise its temperature by 20ºC ?
specific heat capacity of water = 4200 J kg-1 K-1 It is useful to remember that 1cm3 of water has a mass of 1g, and 1 m3 of water has a mass of 1000 kg So 100 cm3 of water has a mass of 100 g or 0.1 kg energy transferred = mass of water heated × specific heat capacity of water × temperature rise = 0.10 × 4200 × 20 = 8,400 J It is also useful to remember that 1 kilojoule, 1 kJ, equals 1,000 J. So the energy transferred is 8.4 kJ. |
Follow me...
|