Expansion and Contraction
Expansion is possible because of increased kinetic energy of the particles involved - this makes them vibrate more and be further apart.
Never say atoms or particles 'expand' to an examiner! It will lose you marks...
Not all materials change dimensions by the same amount when their temperature changes. This is demonstrated by the bi-matallic strip. Bi-metallic strip
The bimetallic strip is usually made of steel and copper, or in some cases brass instead of copper. The strips are joined together throughout their length by riveting, brazing or welding. The different expansion of each metal (when heated to increase (or cooled to decrease)) in temperature forces the flat compound strip to bend one way if heated, and in the opposite direction if cooled below its initial temperature. The metal with the higher coefficient of thermal expansion is on the outer side of the curve when the strip is heated because it increases in size more than the other one - it expands more. The metal with the higher coefficient of thermal expansion is on the inner side when cooled because it decreases in size more than the other one - it contacts more. The metal strips expand in the depth and width dimensions too - but expansion in those directions is so small that it hardly causes any bending. Be careful when reading about this on the web - some sites give the impression that you should talk in terms of 'time' - one metal 'expands quicker' when heated.... implying that the other one given time would catch up. Do not talk in those terms to the examiner. Make sure he knows that you understand that one metal will expand or contract more than the other one when the temperature changes by one degree. This effect is used in a range of mechanical and electrical devices. In some applications the bimetal strip is used in the flat form. In others, it is wrapped into a coil for compactness. The greater length of the coiled version gives improved sensitivity.
The above vid-clip shows a typical high school experiment.
This one shows how a bimetallic switch works - note that when cool it returns to its original state.
|
Follow me...
|



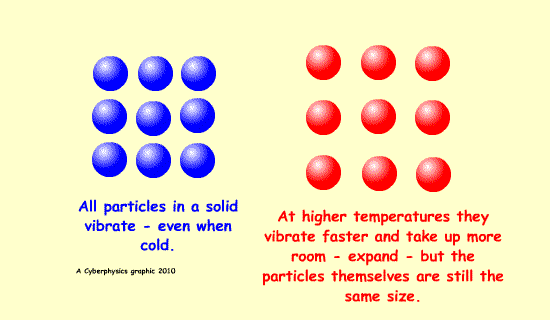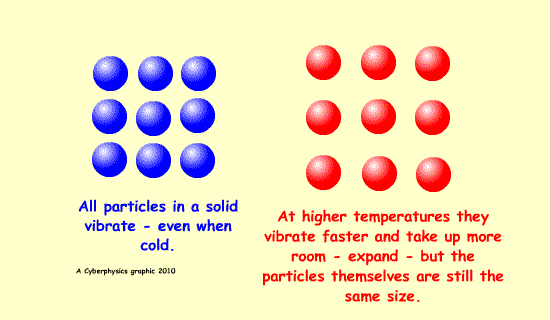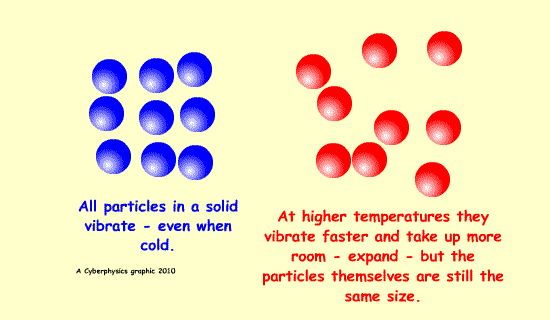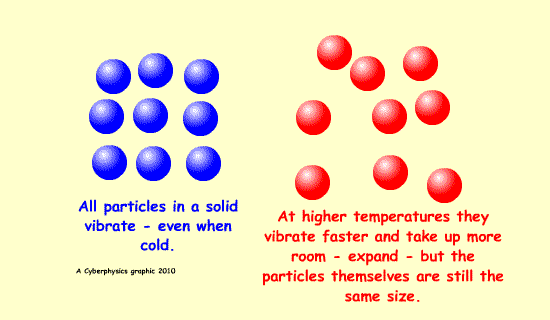
 A bimetallic strip can be used to convert a temperature change into mechanical displacement. The strip is made up of two strips of different metals which expand to a different degree when the temperature changes - they have different expansively - different coefficients of expansion.
A bimetallic strip can be used to convert a temperature change into mechanical displacement. The strip is made up of two strips of different metals which expand to a different degree when the temperature changes - they have different expansively - different coefficients of expansion.
 Questions on expansion
Questions on expansion 


