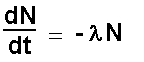|
Question
on Radioactive decay  A speck of uranium dust contains 1014 atoms. It has a mass of 3.95 x 10-8g. The half life of Uranium 238 is 4.5 x 109 years. It is inhaled and lodges in the lung. What is the initial rate of disintegration of the dust spec? 3.95 x 10-8g uranium contains 1014 atoms (how do we know that ?….. see mole section) N0 = 1014 atoms dN/dt = ? half life = 4.5 x 109 years = 4.5 x 109 x 365.25 days = 4.5 x 109 x 365.25 x 24 hours = 4.5 x 109 x 365.25 x 24 x 60 hours = 4.5 x 109 x 365.25 x 24 x 60 x 60 seconds = 1.42 x 1017 seconds l = - ln2/half life = - 0.693/(1.42 x 1017)
= - (- 0.693 /(1.42 x 1017)) x 1014 = 0.693 x 1014 )/ 1.42 x 1017 = 4.9 x 10-4 s-1 = 1.8 h-1 = 42 day-1 = 15,400 year-1 |
Follow me...
|