Specific Heat Capacity and Latent Heat Questions - GCSE standard
Q14.
Bill performs an experiment to find the specific heat capacity of water.
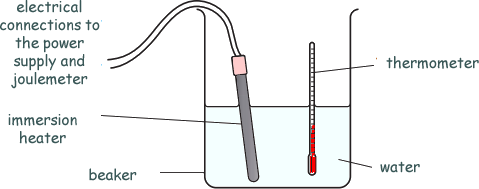
He heats up 1kg of water, using an immersion heater.
He measures the temperature rise and calculates the specific heat capacity of the water.
Attempt |
Energy supplied /J |
Temperature rise /°C |
Specific heat capacity in J/kg°C |
1 |
10000 |
2 |
5000 |
| 2 |
21000 |
4 |
5250 |
3 |
44000 |
8 |
5500 |
(a)
(i) Calculate the mean specific heat capacity.
5000 + 5250 + 5500 = 15750
Mean value = 15750/3 = 15250 J/kgoC 
[1 mark]
(ii) Describe the conclusions that can be drawn from the data.

Any three from: 


 s.h.c. increases with longer heating
s.h.c. increases with longer heating
 specific heat capacity increases with temperature rise
specific heat capacity increases with temperature rise
 specific heat capacity increases with energy supplied
specific heat capacity increases with energy supplied
 temperature rise increases with energy supplied
temperature rise increases with energy supplied
 different amounts of energy were supplied
different amounts of energy were supplied
 all of the s.h.cs. are close together (within 5%)
all of the s.h.cs. are close together (within 5%)
 all of the s.h.c values are within the range 5000 – 5500 J/kgoC
all of the s.h.c values are within the range 5000 – 5500 J/kgoC
 the experiment was repeated three times
the experiment was repeated three times
[3 marks]
(b) The actual value for the specific heat capacity of water is 4200J/kg°C.
(i) Explain why the mean specific heat capacity calculated in (a)(i) is higher than the actual value.
Becuse more energy was recorded as having heated the water than actually did so,  because energy losses occurred as energy was transferred to the environment.
because energy losses occurred as energy was transferred to the environment. 
[2 marks]
(ii) Identify two problems with this experimental procedure and suggest how each one could be solved.
Any two linked answers from:
 Part of the immersion heater is out of the water
Part of the immersion heater is out of the water so make sure the heater is fully in the water or use a larger/deeper beaker.
so make sure the heater is fully in the water or use a larger/deeper beaker. 
 The beaker is not lagged/insulated
The beaker is not lagged/insulated  so lag/insulate the beaker.
so lag/insulate the beaker. 
 There is no lid on the beaker
There is no lid on the beaker  put a lid on the beaker
put a lid on the beaker 
 The temperature rises are quite small
The temperature rises are quite small  apply more energy to the water
apply more energy to the water 
 There is insufficient data
There is insufficient data  so take more readings
so take more readings 
[4 marks]
(10 marks total)







