Specific Heat Capacity and Latent Heat Questions - GCSE standard
Q15.
Bill and Ben design an experiment to find the specific latent heat of water.
They set up their equipment as shown in the diagram.
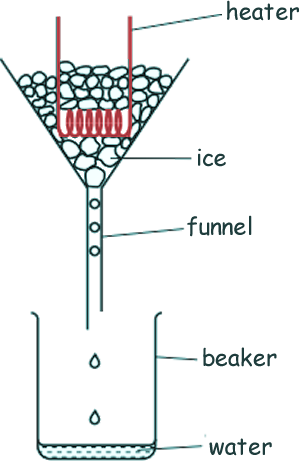
They have access to a power supply, a voltmeter, an ammeter, a stop-clock and a top-pan balance as well as the equipment shown in the diagram.
(a) Explain how Bill and Ben could use this equipment to determine an accurate value for the specific latent heat of water.
[6 marks]

5 - 6 marks |
3 - 4 marks |
1 - 2 marks |
| Level 0: |
No response or no response worthy of credit. |
0 marks |
Examples of physics points that should be made in the response:
 Apply knowledge and understanding of how to use the equipment to find specific latent heat of water.
Apply knowledge and understanding of how to use the equipment to find specific latent heat of water.
For example:
- Measure the initial mass/weight of beaker
- Turn on the heater
- Start timing
- Use the voltmeter, ammeter and stopclock to calculate the energy supplied (E=VIt)
- Turn off the heater
- Stop timing
- Use a balance to measure the mass of the beaker and melted ice
- Subtract the original mass of the beaker to find the mass/weight of the melted ice
- Calculate specific latent heat by dividing energy by mass
 Analyse information and ideas to develop experimental procedures and consider accuracy of the experiment.
Analyse information and ideas to develop experimental procedures and consider accuracy of the experiment.
For example:
- Make sure that the heater is always covered with ice
- Insulate/put lid on the funnel to reduce heat losses
- Make sure that the mass of water produced is sufficiently large to measure accurately – run the experiment for long enough
- Repeat the experiment to minimise (random) errors
(b) The students find that 250g of ice takes 95kJ of energy to change state.
Calculate the specific latent heat of fusion for water in J/kg.

Specific latent heat of fusion = energy/mass 
Energy must be in joules
95 kJ = 95,000J 
Mass must be in kilograms
250g = 0.25 kg 
Specific latent heat of fusion = 95,000/0.25 
Specific latent heat of fusion = 380,000 J/kg 
[5 marks]
(11 marks total)







