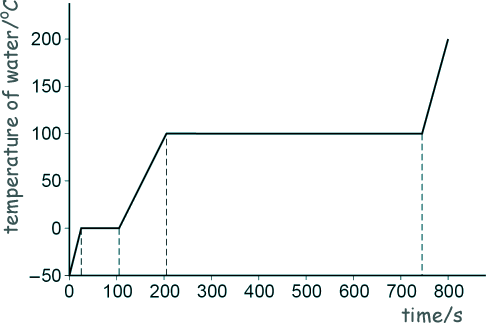
Specific Heat Capacity and Latent Heat Questions - GCSE standard Q17. (a) Andy investigated how the temperature of a lump of ice varied as the ice was heated. He recorded the temperature until the ice melted and then the water produced boiled. The sketch graph below shows Andy’s results. The power output of the heater was constant.
[2 marks]
[2 marks] (b) Nick did the same investigation and recorded the temperature until the water produced boiled. In the Nick's investigation more thermal energy was transferred to the surroundings. Describe two ways the results of Nick's experiment would differ from those shown in Andy's sketch graph. Any two from:
[2 marks] (c) When the water was boiling, 30 g of water turned into steam. The energy transferred to the water was 69 kJ. Calculate the specific latent heat of vaporisation of water. 30 g = 30 x 10-3 kg 69 kJ = 69 x 103 J Energy = mass x specific latent heat of vapourisation E = m l l = E/m l = 69 x 103/(30 x 10-3) l = 2.3 x 106 J/kg [5 marks] (11 marks total) |
Follow me...
|