Specific Heat Capacity and Latent Heat Questions - GCSE standard
Q16.
A student investigated the three states of matter.
The arrangement of particles in each of the three states of matter are different.
(a) Name the states of matter represented by the particle arrangements indicated in diagrams A, B and C.
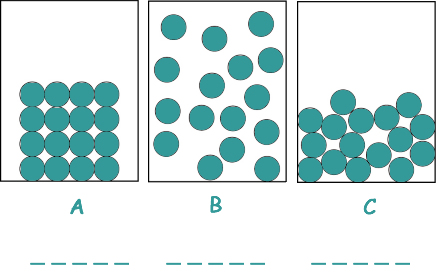
[2 marks]
(b) A large lump of ice was heated and observed as it changed state.
The graph below shows how the temperature varied with time.
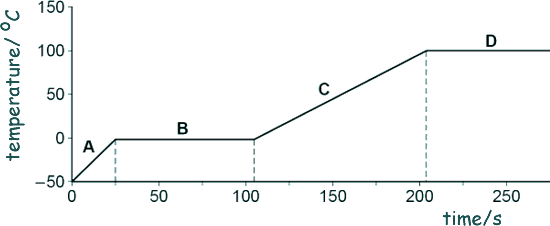
(i) Which letter shows the portion of the graph when the ice was melting?
[1 mark]
(ii) Which letter shows the portion of the graph when the water was boiling?
[1 mark]
(c) Name the property of the water particles that changes as the temperature of the water increases?
[1 mark]
(d) Calculate the thermal energy needed to melt 250 g of ice at 0 °C, given that the specific latent heat of fusion of water = 334 000 J/kg
[3 marks]
(e) What is the name given to the process that occurs when a substance is heated and changes directly from a solid to a gas.
(1 mark)
(9 marks total)









