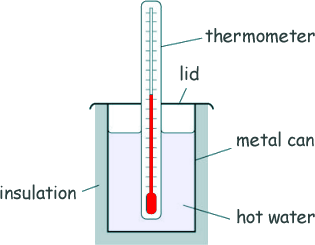
Specific Heat Capacity and Latent Heat Questions - GCSE standard Q18. Kayjay investigated the insulating properties of different materials. The diagram shows some of the equipment used by her.
This is the method she used:
(a) Identify the independent variable and the dependent variable in this investigation. [2 marks] (b) Kayjay used two different types of thermometer to measure the temperature changes. The diagram below shows a reading on each thermometer.
[1 mark]
[1 mark] (c) For one type of insulating material, the temperature of the water decreased from 85.0 °C to 65.0 °C. The energy transferred from the water was 10.5 kJ. Given that the specific heat capacity of water is 4200 J/kg °C, calculate the mass of water in the can. [3 marks] (d) The table below shows the results for two insulating materials.
Explain how these results can be used to compare the thermal conductivity of the two materials. [2 marks] (9 marks total) |
Follow me...
|