A level: Kinetic Theory Questions
Q10.
(a)
(i) Write down the equation of state for n moles of an ideal gas.

pV = nRT 
(ii) The molecular kinetic theory leads to the derivation of the equation pV = 1/3 Nm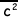 , where the symbols have their usual meaning.
, where the symbols have their usual meaning.
State three assumptions that are made in this derivation.
Any three of the following:
 all particles identical or have same mass
all particles identical or have same mass 
 collisions of gas molecules are elastic
collisions of gas molecules are elastic 
 inter molecular forces are negligible (except during collisions)
inter molecular forces are negligible (except during collisions) 
 volume of molecules is negligible (compared to volume of container)
volume of molecules is negligible (compared to volume of container) 
 time of collisions is negligible
time of collisions is negligible 
 motion of molecules is random
motion of molecules is random 
 large number of molecules present (therefore statistical analysis applies)
large number of molecules present (therefore statistical analysis applies) 
 monamatic gas (point particles)
monamatic gas (point particles) 
 Newtonian mechanics applies
Newtonian mechanics applies 
(4 marks)
(b) Calculate the average kinetic energy of a gas molecule of an ideal gas at a temperature of 20 °C.
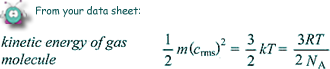
T = 273 + 20 = 293K 
Ek = 3/2 kT
Ek = 1.5 x 1.38 × 10–23 x 293 
Ek = 6.1 x 10-21 J 
(3 marks)
(c) Two different gases at the same temperature have molecules with different mean square speeds. Explain why this is possible.
Ek is the same at a given temperature.
Kinetic energy is related to the mass and square of speed, therefore if the masses of the molecules are different the mean square speeds must be different, as the product of the mass and mean square speed must be kept constant.
(2 marks)
(Total 9 marks)