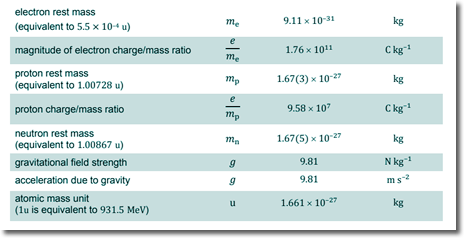
E = mc2 - mass energy conversion Q5. (a) Calculate the binding energy, in MeV, of a nucleus of the nuclear mass of Binding energy = Mass of individual nucleons - Mass of nucleus Mass defect Δm = Z mp + N mn – MCo 27 protons 59 - 27 = 32 neutrons
EBinding = (27 x 1.00728 + 32 x 1.00867) - 58.93320 EBinding = (27.19656 + 32.27744) - 58.93320 EBinding = 59.474 - 58.93320 EBinding = 0.5408u Now, 1u = 931.5 MeV ∴ EBinding = 931.5 x 0.5408 MeV EBinding = 503.8 MeV [3 marks] (b) A nucleus of iron It decays by β− emission, followed by the emission of γ-radiation as the The total energy released when the The
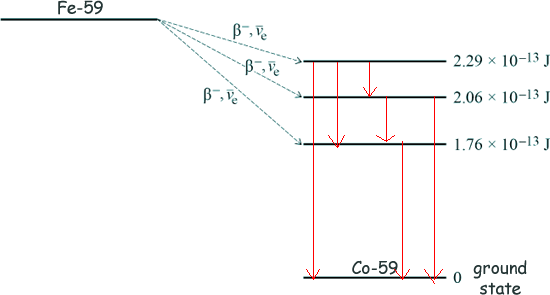
Calculate the maximum possible kinetic energy, in MeV, of the β− particle emitted when the Maximum kinetic energy will remain if the beta particle transfers to the 1.76 x 10-13 J state. 2.52 × 10−13 - 1.76 x 10-13 = 0.76 x 10-13J Energy = 7.6 x 10-14J 1.6 x 10-13 J = 1 MeV Energy = 7.6 x 10-14/(1.6 x 10-13 ) Energy = 0.48 MeV [2 marks] (c) Following the production of excited states of
[1 mark]
[3 marks] (Total 9 marks) |
Follow me...
|