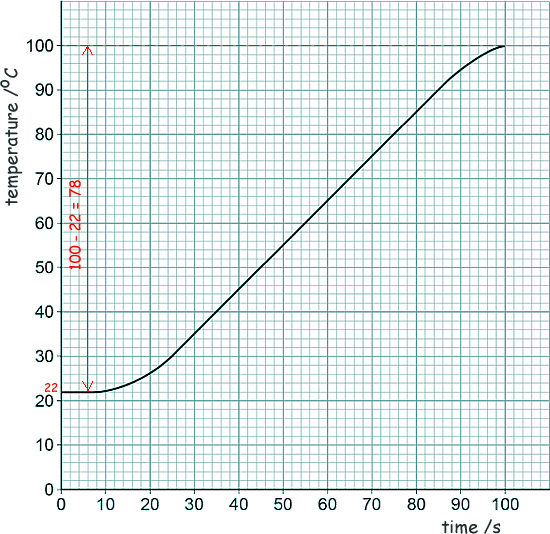
Specific Heat Capacity and Latent Heat Questions - GCSE standard Q13. An electric kettle was switched on. The graph shows how the temperature of the water inside the kettle changed.
(a) When the kettle was switched on the temperature of the water did not immediately start to increase. Suggest one reason why. The heating element of the kettle takes time to heat up itself. [1 mark] (b) The energy transferred to the water in 100 seconds was 155,000 J. Given that the specific heat capacity of water = 4200 J/kg °C, use the graph to determine the mass of water in the kettle. Give your answer to 2 significant figures. Δθ = 78 °C E = mc Δθ 155,000 = m × 4200 × 78 m = 155,000 / (4200 × 78) m = 0.4731 kg m = 0.47 kg (to 2 sf) [5 marks] (c) Explain how the straight section of the line graph can be used to calculate the useful power output of the kettle. Gradient of the graph = ∆θ/t
E = mc Δθ
E/t = mc Δθ/t
E/t = P
P = mc x gradient of the graph
[3 marks] (9 marks total) |
Follow me...
|