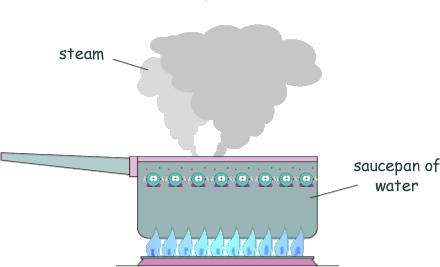
Specific Heat Capacity and Latent Heat Questions - GCSE standard Q8. The diagram shows water being heated. Eventually the water changed into steam.
(a)
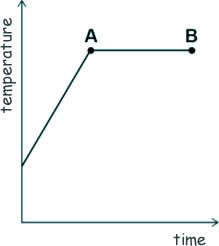
[2 marks] (b) The sketch-graph shows how the temperature of the water varied with time.
What is the name of the process that is taking place between points A and B? Give a reason for your answer. [2 marks] (c) A mass of 63 g of water was turned into steam. The specific latent heat of vaporisation of water is 2.26 MJ/kg Calculate the thermal energy transferred to the water to turn it into steam. [4 marks] (d) The mass of the steam was 0.063 kg. The volume of the steam was 0.105 m3 Calculate the density of steam. [3 marks] (Total 11 marks) |
Follow me...
|