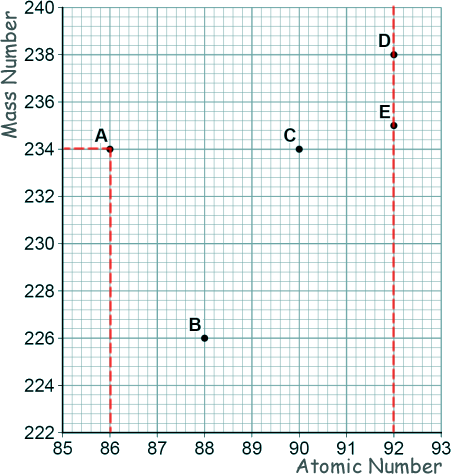
GCSE Questions: Radioactivity Q19. (a) The graph shows a plot for the mass number and the atomic number for the nuclei of five different atoms.
[1 mark]
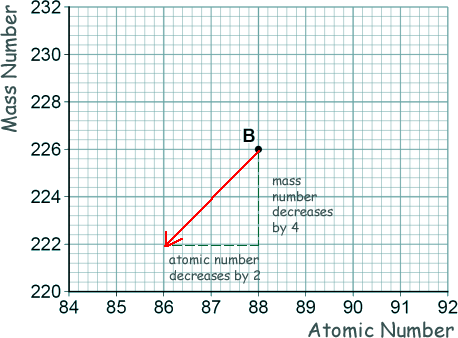
[1 mark] (b) Nucleus B decays by emitting an alpha particle.
Draw an arrow on the diagram to represent the alpha decay.
[2 marks] (c) What is meant by the 'random nature of radioactive decay'? That you can't predict which nucleus will decay next or when a (particular) nucleus will decay. [1 mark] (d) A polonium (Po) nucleus decays by emitting an alpha particle and forming a lead (Pb) nucleus. Po → Pb + α The lead (Pb) nucleus then decays by emitting a beta particle and forms a bismuth (Bi) nucleus. Pb → Bi + β The bismuth (Bi) nucleus then decays by emitting a beta particle and forms a polonium (Po) nucleus. Bi → Po + β Explain how these three decays result in a nucleus of the original element, polonium. The identity of an element is its atomic number - the number of protons in the nucleus. Alpha decay reduces the number of protons in a nucleus by 2. Beta decay increases the number of protons in a nucleus by 1. Two beta decays therefore replace the protons lost by an alpha decay resulting in the same number of protons as at the start of the decay sequence and therefore the same element (although a different isotope of it). [3 marks] (Total 8 marks) |
Follow me...
|