GCSE Questions: Radioactivity
Q7.
(a) The graph shows how a sample of barium-143, a radioactive isotope with a short half-life, decays with time.
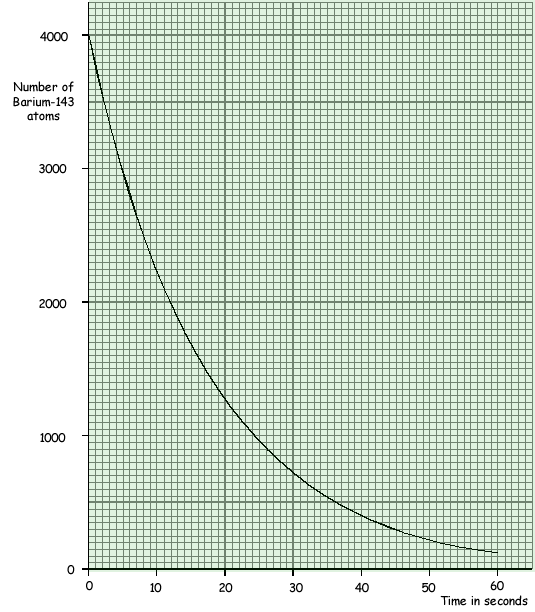
(i) What is meant by the term isotope?
(1 mark)
(ii) What is meant by the term half-life?
(1 mark)
(iii) Use the graph to find the half-life of barium-143.
(1 mark)
(b) Humans take in the radioactive isotope carbon-14 from their food. After their death, the proportion of carbon-14 in their bones can be used to tell how long it is since they died. Carbon-14 has a half-life of 5700 years.
(i) A bone in a living human contains 80 units of carbon-14. An identical bone taken from a skeleton found in an ancient burial ground contains 5 units of carbon-14.
 Calculate the age of the skeleton.
Calculate the age of the skeleton.
 Show clearly how you work out your answer.
Show clearly how you work out your answer.
(2 marks)
(ii) Why is carbon-14 unsuitable for dating a skeleton believed to be about 150 years old?
(1 mark)
(c) The increased industrial use of radioactive materials is leading to increased amounts of radioactive waste.
Some people suggest that radioactive liquid waste can be mixed with water and then safely dumped at sea.
 Do you agree with this suggestion?
Do you agree with this suggestion?
 Explain the reason for your answer.
Explain the reason for your answer.
(3 marks)
(Total 9 marks)



