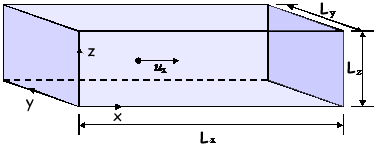
A level: Kinetic Theory Questions Q1. A single gas molecule of mass m is moving in a rectangular box with a velocity of ux in the positive x-direction, as shown in the diagram. The molecule moves backwards and forwards in the box, striking the end faces normally and making elastic collisions.
(a)
(2 marks) (b) If it is assumed that the box contains N identical molecules, each of mass m, all moving parallel to the x-direction with speed ux and making elastic collisions at the ends, show that the average force, F, on the shaded face is given by F = Nmux2/Lx (2 marks) (c) In a better model of molecular motion in gases, molecules of mean square speed are assumed to move randomly in the box.
(5 marks) (Total 9 marks) |
Follow me...
|