Particle Physics Questions : Multiple Choice
Q1. What are the numbers of hadrons, baryons and mesons in an atom of
There are 3 protons and 4 neutrons - which are baryons and hadrons There are no mesons! Q2. A radioactive nucleus emits a β– particle then an α-particle and finally another β– particle. The final nuclide is:
Beta emission a neutron changes to a proton - two betas so the proton number increases by two. Alpha emission means two protons leave the nucleus (along with two neutrons) so the proton number decreases by two. Overall effect - no change in proton number - same element!
Q3. The nucleus of What is the product nucleus?
Q4. Electron capture can be represented by the equation: p + e- → X + YWhich row correctly identifies X and Y?
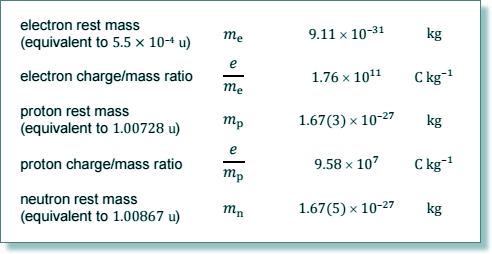
Q5. A calcium ion is formed by removing two electrons from an atom of What is the specific charge of the calcium ion?
Q6. The table below links exchange particles with nuclear processes. Select the line that does not give the correct exchange particle for the process.
Q7. Select the line that gives the correct category and quark combination for the particle.
Q8. Select the incorrect statement about muons from the following.
Q9. Which of the following nuclei has the smallest specific charge?
Q10.
|
Follow me...
|