The Photoelectric
Effect
- evidence of light as a particle -
 |
Click here to read about the photoelectric effect experiment and a bit of history of its discovery. |
 |
 |
Click here to go to a page of videoclip explanations |
 |
Click here to go to the graph |
 |
Click here to go to stopping potential |
 |
Click here to go to A level standard questions on this topic |
What is the Photoelectric Effect?
 The
photoelectric effect is the emission of electrons by substances,
especially metals, when light falls on their surfaces. The
photoelectric effect is the emission of electrons by substances,
especially metals, when light falls on their surfaces.
 If you illuminate a metallic surface with photons of electromagnetic radiation above a threshold frequency, the photons are absorbed and electrons are emitted from the surface . If you illuminate a metallic surface with photons of electromagnetic radiation above a threshold frequency, the photons are absorbed and electrons are emitted from the surface .
 No electrons are emitted for radiation with a frequency below that of the threshold, as the electrons are unable to gain sufficient energy to overcome the electrostatic barrier of the work function of the metal. No electrons are emitted for radiation with a frequency below that of the threshold, as the electrons are unable to gain sufficient energy to overcome the electrostatic barrier of the work function of the metal.
|
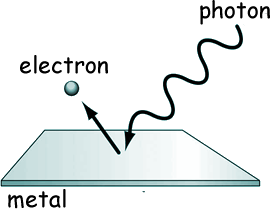 |
 The photon frequency required to make this happen is unique to the type of surface and material the radiation fall on.
The photon frequency required to make this happen is unique to the type of surface and material the radiation fall on.
 The energy of the photon is all absorbed by the electron and if it is sufficient to free the electron from the hold of the metal lattice, the electron can escape from the material. The energy supplied above the work function will give a finite kinetic energy to the escaped electron.
The energy of the photon is all absorbed by the electron and if it is sufficient to free the electron from the hold of the metal lattice, the electron can escape from the material. The energy supplied above the work function will give a finite kinetic energy to the escaped electron.
 A single photon can only eject a single electron, as the energy of one photon may only be absorbed by one electron. A single photon can only eject a single electron, as the energy of one photon may only be absorbed by one electron.
|
 |
 Each electron can absorb energy by absorbing one photon when irradiated by electromagnetic energy, but as they adhere to an "all or nothing" code of conduct, all of the energy from that one photon must be absorbed and used to free one electron from atomic binding, or the energy must be re-emitted - the photon must be rejected. When a photon of sufficient energy to allow the electron to escape the metal lattice is absorbed, some of the energy is used to liberate it from the atom, the remainder contributes to the electron's kinetic (moving) energy as a free particle.
Each electron can absorb energy by absorbing one photon when irradiated by electromagnetic energy, but as they adhere to an "all or nothing" code of conduct, all of the energy from that one photon must be absorbed and used to free one electron from atomic binding, or the energy must be re-emitted - the photon must be rejected. When a photon of sufficient energy to allow the electron to escape the metal lattice is absorbed, some of the energy is used to liberate it from the atom, the remainder contributes to the electron's kinetic (moving) energy as a free particle.
 The electrons that are emitted are often called photoelectrons.
The electrons that are emitted are often called photoelectrons.
 Each photon has a specific energy related to its wavelength, such that photons
of short wavelength (blue light) carry more energy than long wavelength
(red light) photons.
Each photon has a specific energy related to its wavelength, such that photons
of short wavelength (blue light) carry more energy than long wavelength
(red light) photons.
E = hf = hc/λ
 To release an electron from
a metal plate requires a energy above a certain value. That energy value is
called the 'work function energy'. It is the energy required
to do work against the structure of the metal that the electron is
part of.
To release an electron from
a metal plate requires a energy above a certain value. That energy value is
called the 'work function energy'. It is the energy required
to do work against the structure of the metal that the electron is
part of.
Freeing the electrons
 An electron on the surface of the metal (the least tightly
held one in the structure) is bound to the metal by that energy and
to release it you have to input that amount of energy. Each metal has
a different structure and so has been found to have a different 'work function'.
Those that hold on to their electrons lightly have low work functions
those that hold on tightly have a high one!
An electron on the surface of the metal (the least tightly
held one in the structure) is bound to the metal by that energy and
to release it you have to input that amount of energy. Each metal has
a different structure and so has been found to have a different 'work function'.
Those that hold on to their electrons lightly have low work functions
those that hold on tightly have a high one!
 The electrons can not absorb
more than one photon to escape from the surface, they can not therefore
absorb one quanta and then another to make up the required amount -
it is as if they can only embrace one quantum at a time. If the quantum
absorbed is not of sufficient energy the electron can not break free.
So 'escape energy' can only be transferred by a photon of energy equal
or greater than that minimum threshold energy (i.e. the wavelength of
the light had to be a sufficiently short).
The electrons can not absorb
more than one photon to escape from the surface, they can not therefore
absorb one quanta and then another to make up the required amount -
it is as if they can only embrace one quantum at a time. If the quantum
absorbed is not of sufficient energy the electron can not break free.
So 'escape energy' can only be transferred by a photon of energy equal
or greater than that minimum threshold energy (i.e. the wavelength of
the light had to be a sufficiently short).
 So, if each photon of blue light
releases an electron but all red photons were too weak, the result
is that no matter how much red light is shone on the metal plate, there
will be no current.
So, if each photon of blue light
releases an electron but all red photons were too weak, the result
is that no matter how much red light is shone on the metal plate, there
will be no current.
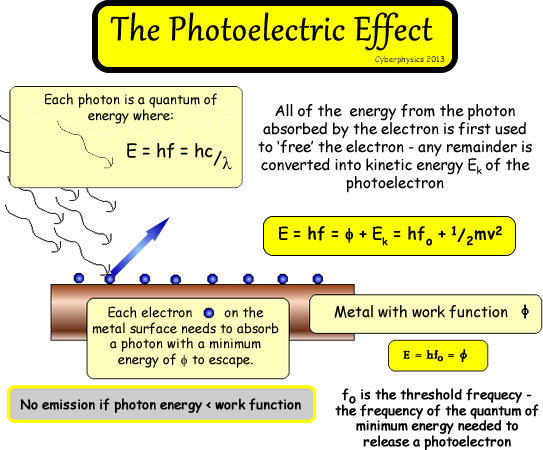
 If the photon absorbed is
of a higher energy than required to just free the electron, the remainder
becomes kinetic energy of the free electron. Therefore the maximum kinetic
energy of the electrons released depends on the work function
If the photon absorbed is
of a higher energy than required to just free the electron, the remainder
becomes kinetic energy of the free electron. Therefore the maximum kinetic
energy of the electrons released depends on the work function  of the metal and the frequency of the light shone onto it! The most losely held electron (one on the surface) will have the most energy left over and will have the maximum vale - the others will have lower amounts of KE as they need more to escape.
of the metal and the frequency of the light shone onto it! The most losely held electron (one on the surface) will have the most energy left over and will have the maximum vale - the others will have lower amounts of KE as they need more to escape.
E
= hf = Φ + Ek (MAX)
This
equation means that:
 A photon (E = hf) absorbed by the electron (in entirety - because it cannot share
its energy among several!) is used to provide energy for the electron
to escape from the metal Φ
and any excess energy is converted into kinetic
energy.
A photon (E = hf) absorbed by the electron (in entirety - because it cannot share
its energy among several!) is used to provide energy for the electron
to escape from the metal Φ
and any excess energy is converted into kinetic
energy.
 The MAX is there because the electrons with the most energy
left over for KE will be those on the surface, but some a little deeper
will also escape but need to use more of the energy to do work against
the metal's hold on them! So, in reality a range of energies will be
observed.
The MAX is there because the electrons with the most energy
left over for KE will be those on the surface, but some a little deeper
will also escape but need to use more of the energy to do work against
the metal's hold on them! So, in reality a range of energies will be
observed.
 Sketch
a graph of the kinetic energy of the 'freed' photoelectrons against frequency
using the above equation - click here
for the solution...
Sketch
a graph of the kinetic energy of the 'freed' photoelectrons against frequency
using the above equation - click here
for the solution...
This phenomenon
could not be understood without the concept of light as being particle-like
- a photon, a quantum amount of light energy for a particular frequency.
If light were simple a wave-like phenomenon then increasing the intensity
and thereby increasing the total energy falling on the surface would
be expected to eventually provide enough energy to release electrons
no matter what the frequency. Furthermore, in the classical picture
one would expect the maximum energy of the emitted electrons to depend
on the intensity of the light -- but it does not.
So this is evidence
that light behaves as if it were a particle.
Summary of photoelectric emission
 For a given metal and frequency of incident radiation, the number of photoelectrons ejected per second is directly proportional to the intensity of the incident light.
For a given metal and frequency of incident radiation, the number of photoelectrons ejected per second is directly proportional to the intensity of the incident light.
 For a given metal, there exists a certain minimum frequency of incident radiation below which no emission of photoelectrons takes place. This frequency is called the threshold frequency.
For a given metal, there exists a certain minimum frequency of incident radiation below which no emission of photoelectrons takes place. This frequency is called the threshold frequency.
 Above the threshold frequency, the maximum kinetic energy of the emitted photoelectron is independent of the intensity of the incident light but depends only upon the frequency (or wavelength) of the incident light.
Above the threshold frequency, the maximum kinetic energy of the emitted photoelectron is independent of the intensity of the incident light but depends only upon the frequency (or wavelength) of the incident light.
 The time lag between the incidence of radiation and the emission of a photoelectron is very small, less then 10-9 second.
The time lag between the incidence of radiation and the emission of a photoelectron is very small, less then 10-9 second.
 This phenomenon is evidence of the particular nature of light.
This phenomenon is evidence of the particular nature of light.
------------------------------------------------------------------------------------------------
 Here is a Java
CheerpJ simulation for you to play with!
Here is a Java
CheerpJ simulation for you to play with!
It is hosted by Colorado University.
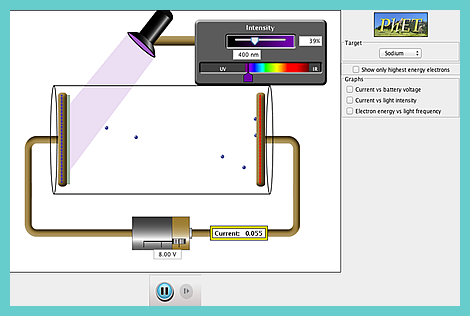
It will help you to:
 Visualize and describe the photoelectric effect experiment.
Visualize and describe the photoelectric effect experiment.
 Correctly predict the results of experiments of the photoelectric effect: e.g.
Correctly predict the results of experiments of the photoelectric effect: e.g.
- how changing the intensity of light will affect the current and the energy of electrons,
- how changing the wavelength of light will affect the current and the energy of electrons,
- how changing the voltage of light will affect the current and the energy of electrons,
- how changing the material of the target will affect the current and the energy of electrons.
 Describe how these results lead to the photon model of light: e.g. argue that only a photon model of light can explain why, when light is shining on the metal but there is no current, increasing the frequency will lead to a current, but increasing the intensity of light or the voltage between the plates will not.
Describe how these results lead to the photon model of light: e.g. argue that only a photon model of light can explain why, when light is shining on the metal but there is no current, increasing the frequency will lead to a current, but increasing the intensity of light or the voltage between the plates will not.
 |
Ready for some questions?
Click on the icon - questions and answers at A level standard await you!
For more topics that have practice questions associated with them see the upper left menu bar
|








 Here is a
Here is a 




