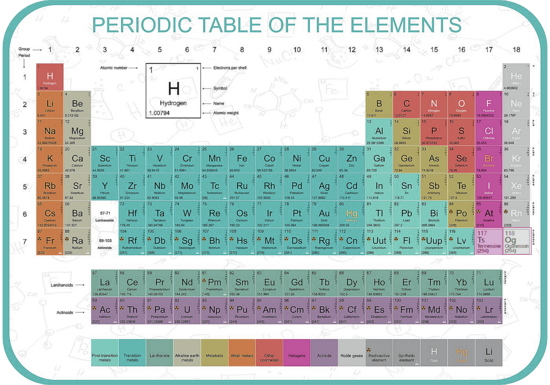
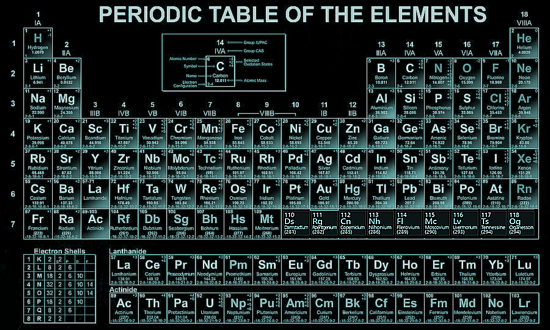
The Periodic Table The periodic table lists the chemical elements in a table - arranged in atomic number - in a way that indicates their electron configuration. It is the electronic configuration that explains recurring chemical properties in groups of elements so the ones with similar chemical behaviour are arranged to fall into vertical columns - the vertical columns are called groups. Under a 1988 international naming convention, the groups are now numbered numerically from 1 to 18 from the leftmost column (the alkali metals) to the rightmost column (the noble gases).
Periodic trends are observed across the seven rows of the table - each row is call a period. Generally metals are on the left of each period and nonmetals are on the right. The elements from atomic numbers 1 (hydrogen) to 118 (oganesson) have all been discovered or synthesized, completing seven full rows of the periodic table. Six groups have accepted names as well as assigned numbers:
Also displayed are four simple rectangular areas or blocks associated with the filling of different atomic orbitals. The first 94 elements, hydrogen to plutonium, all occur naturally, though some are found only in trace amounts and a few were discovered in nature only after having first been synthesized. Elements 95 to 118 have only been synthesized in laboratories, nuclear reactors, or nuclear explosions. The synthesis of elements having higher atomic numbers is currently being pursued: these elements would begin an eighth row, and theoretical work has been done to suggest possible candidates for this extension. Dmitri MendeleevThe Russian chemist Dmitri Mendeleev published the first recognizable periodic table in 1869, developed mainly to illustrate periodic trends of the then-known elements. He also predicted some properties of unidentified elements that were expected to fill gaps within the table. Most of his forecasts soon proved to be correct, culminating with the discovery of gallium and germanium in 1875 and 1886 respectively, which corroborated his predictions. Mendeleev's idea has been slowly expanded and refined with the discovery or synthesis of further new elements and the development of new theoretical models to explain chemical behaviour. |
Follow me...
|