Electrical Conduction
Metals have outer shells with loosely held electrons in them that they are happy to allow to wander throughout the metal. They therefore make excellent conductors. Non-metals do not have those 'free' electrons so they do not conduct as well and are called insulators.
Molten metals conduct electricity, so do ionic solutions as they have charged particles (ions) that can move.
|
Follow me...
|


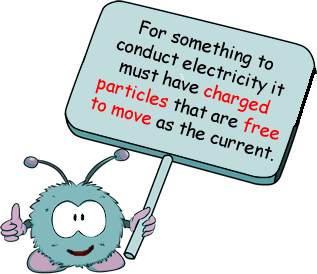 For something to conduct electricity it must have charged particles that are free to move as the current.
For something to conduct electricity it must have charged particles that are free to move as the current.


