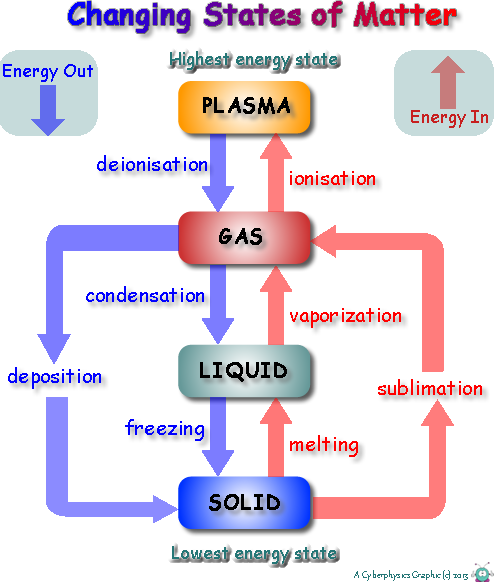
Changing State We have seen that we can think of everything as being made up of particles, and that how these particles behave can be used to explain the differences between each of the three states of matter that we study at GCSE Level. Internal Energy of a systemEnergy is stored inside a system by the particles (atoms and molecules) that make up the system. This is called internal energy. Our particle model defines internal energy as having two components: kinetic energy and internal potential energy (
In order to 'change state' internal energy is either 'taken in' or 'given out'.
When energy is taken in to change state it is used to 'free' particles from their neighbouring particles. It increases their internal potential energy. (It does not increase kinetic energy... therefore does not cause a rise in temperature).
We think of 'changing state' as only occurring at boiling point and melting point temperatures, but that is not the case.
|
Follow me...
|