RMS Speed Questions
 Standard Temperature and Pressure (STP)
Standard Temperature and Pressure (STP)
Q1. Calculate the RMS speed of air molecules in a container in which the pressure is 1.0 x 105 Pa and the density of air is 1.3 kg m-3.
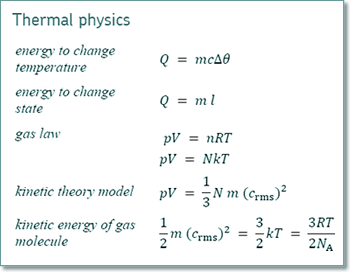
ρ = Nm/V and V = Nm/ρ
pV = 1/3 Nm (crms)2
pNm/ρ = 1/3 Nm (crms)2
p/ρ = 1/3 (crms)2
(crms)2 = 3p/ρ
crms = √(3p/ρ)
crms = √(3.0 x 105/1.3 )
crms = 480 ms-1
Q2. The following table shows the distribution of the speeds of 20 particles:
| Speed/ms-1 |
10 |
20 |
30 |
40 |
50 |
60 |
| Number of particles |
1 |
3 |
8 |
5 |
2 |
1 |
| Squared Speed/m
2
s2 |
100 |
400 |
900 |
1600 |
2500 |
3600 |
Find
(a) the most probable speed,
30 m/s
(b) the average speed,
= (10 + 3 x 20 + 8 x 30 + 5 x 40 + 2 x 50 + 60)/20
= (10 + 60 + 240 + 200 + 100 + 60)/20
= 670/20 = 33.5 m/s
= 34 m/s
(c) the RMS speed.
= (100 + 3 x 400 + 8 x 900 + 5 x 1600 + 2 x 2500 x 3600)/20
= (100 + 1200 + 7200 + 8000 + 5000 + 3600)/20
= 25100/20
= 1255
35.4 m/s
35 m/s
Q3. The RMS speed of helium at STP is 1.30 km s-1. Calculate the density of helium at STP.
ρ = Nm/V and V = Nm/ρ
pV = 1/3 Nm (crms)2
pNm/ρ = 1/3 Nm (crms)2
p/ρ = 1/3 (crms)2
ρ = 3p/ (crms)2
crms = 1.30 km s-1 = 1300 m s-1
ρ = 3 x 1.01 x 105/ (1300)2
ρ = 0.179 kg m-3
Q4. The RMS speed of nitrogen molecules at 127oC is 600 ms-1. Calculate the RMS speed at 1127oC.
½m (crms)2 = 3/2kT
so (crms)2 ∝ T
T1 = 127 + 273 = 400K
T2 = 1127 + 273 = 1400K
6002/400 = x2/1400
x2 = 6002 x 1400/400 = 1 260 000
x = 1120 m/s
Q5. If the density of nitrogen at STP is 1.25 kg m-3, calculate the RMS speed of nitrogen at 227 °C.
ρ = Nm/V and V = Nm/ρ
pV = 1/3 Nm (crms)2
pNm/ρ = 1/3 Nm (crms)2
p/ρ = 1/3 (crms)2
(crms)2 = 3p/ρ
At STP: (crms)2 = 3.03 x 105/1.25
½m (crms)2 = 3/2kT
so (crms)2 ∝ T
T1 = 0 + 273 = 273K
T2 = 227 + 273 = 500K
3.03 x 105/1.25 x 273 = x2/500
x2 = 500 x 3.03 x 105/1.25 x 273
x = 666 m/s
Q6. Calculate the temperature in degrees Celsius at which the RMS speed of oxygen molecules is twice as great as their RMS speed at 27 °C.
½m (crms)2 = 3/2kT
so (crms)2 ∝ T
T1 = 27 + 273 = 300K
(crms)2/300 = (2crms)2/T2
(crms)2/300 = 4(crms)2/T2
1/300 = 4/T2
T2 = 1200 K
T2 = 1200 - 273 = 927oC


