Characteristic X-rays 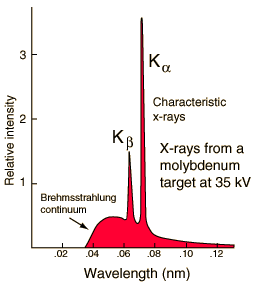
Characteristic X-rays are produced by transitions of orbital electrons from outer to inner shells. Bombarding electrons can release electrons from inner energy level orbits. Higher electrons can then fall into the vacancy and if the energy gap between the levels is great enough X-rays will be produced. Since the electron binding energy for every element is different, the characteristic X-rays produced in the various elements are also different. This type of X-radiation is called characteristic radiation because it has precisely fixed,or discrete, energies and that these energies are characteristic of the differences between electron binding energies of a particular element. The effective energy characteristic X-rays increases with increasing atomic number of the target element. The two sharp peaks in the graph are characteristic X-rays which occur when vacancies are produced in K-shell of the atom and electrons drop down from above to fill the gap. The X-rays produced by transitions from L to K levels are called K-alpha x-rays, and those from M to K transition are called K-beta x-rays. Transitions to the L-shell are designated as L x-rays. The graph also shows the "brehmsstrahlung" radiation which forms the base for the two sharp peaks. Example to calculate the emitted x-ray energy: For tungsten, K electrons have binding energies of 69.5 keV, and L electrons are bound by 12.1 keV. A K-shell electron is removed from a tungsten atom and is replaced by an L shell electron. What is the energy of the characteristic X-ray that is emitted (in keV)? Answer: 57.4 keV (the difference in the energies of the shells).
|
Follow me...
|





