Radioactive Decay
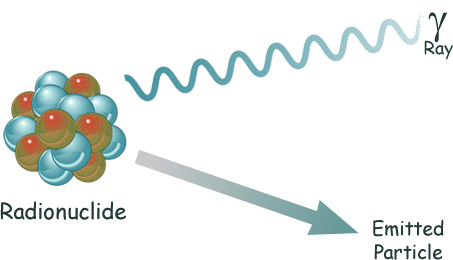
Radioactive decay
is the process of the random, spontaneous transformation of a radionuclide by the emission of nuclear radiation.
 It is 'spontaneous' because we cannot 'make it happen' by changing the conditions the sample is in - such as making it hotter or putting it under pressure. Similarly we cannot stop it happening - that is why nuclear waste is such a problem!
It is 'spontaneous' because we cannot 'make it happen' by changing the conditions the sample is in - such as making it hotter or putting it under pressure. Similarly we cannot stop it happening - that is why nuclear waste is such a problem!
 The
emission of the nuclear radiation is a purely random event. It cannot be predicted exactly when
an atom will decay, only that a certain number will decay
in a given time. The mathematics of probability is used for
this requires a large number of atoms to be considered. (See half life and radioactive
decay series).
The
emission of the nuclear radiation is a purely random event. It cannot be predicted exactly when
an atom will decay, only that a certain number will decay
in a given time. The mathematics of probability is used for
this requires a large number of atoms to be considered. (See half life and radioactive
decay series).
 Radioactive
nuclear decay occurs whenever a nucleus is in an energy-state
that is not the lowest possible for its nucleon
number. This state may occur naturally (which essentially
means that it was created in that state when formed within a
star) or by artificial means (neutron or photon irradiation).
Radioactive
nuclear decay occurs whenever a nucleus is in an energy-state
that is not the lowest possible for its nucleon
number. This state may occur naturally (which essentially
means that it was created in that state when formed within a
star) or by artificial means (neutron or photon irradiation).

 The nucleus remaining is called the decay
product or daughter nucleus.
The nucleus remaining is called the decay
product or daughter nucleus.
 The rate of decay depends on the number of undecayed nuclei present,
so with each decay event there is a decrease in the activity of a radioactive sample.
The rate of decay depends on the number of undecayed nuclei present,
so with each decay event there is a decrease in the activity of a radioactive sample.
 See here for the five types of nuclear radiation.
See here for the five types of nuclear radiation.
 See here for the dangers of nuclear radiation.
See here for the dangers of nuclear radiation.

For a more mathematical look at this - see here


