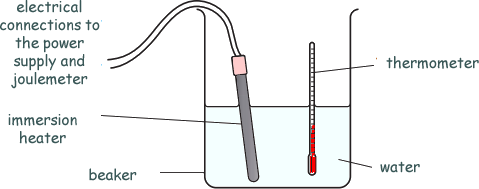
Specific Heat Capacity and Latent Heat Questions - GCSE standard Q14. Bill performs an experiment to find the specific heat capacity of water.
He heats up 1kg of water, using an immersion heater. He measures the temperature rise and calculates the specific heat capacity of the water.
(a)
[1 mark]
[3 marks] (b) The actual value for the specific heat capacity of water is 4200J/kg°C.
[2 marks]
[4 marks] (10 marks total) |
Follow me...
|