Quantum Phenomena - discrete energy levels for electrons
Q3.
(a) Line spectra were observed before they could be explained by theory. We now know that photons of characteristic frequency are emitted when the vapour of an element is bombarded by energetic electrons.
The spectrum of the light emitted contains lines, each of a definite wavelength.
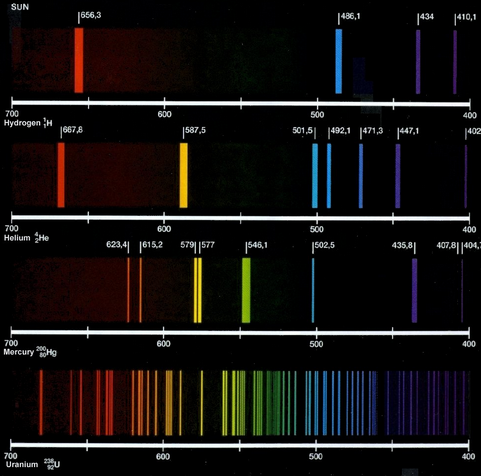
Explain how
• the bombarding electrons cause the atoms of the vapour to emit photons
• the existence of a spectrum consisting of lines of a definite frequency supports the view that atoms have discrete energy levels.
The high energy electrons bombard atoms of vapour, interact with atomic orbital electrons and give energy to to those electrons.  If an orbital electron recieves enough energy to move to a higher energy and there is a vacant state for it, it will be 'prmoted' to the higher energy level.
If an orbital electron recieves enough energy to move to a higher energy and there is a vacant state for it, it will be 'prmoted' to the higher energy level.  Such an electron is called an 'excited electron'.
Such an electron is called an 'excited electron'.
Excited electrons move down to lower energy levels, losing energy by emitting photons  of energy specific to the energy difference between the energy levels.
of energy specific to the energy difference between the energy levels.  Photons have energy that is calculated by E=hf, therefore photons of characteristic frequencies are emitted from atoms of a particular element.
Photons have energy that is calculated by E=hf, therefore photons of characteristic frequencies are emitted from atoms of a particular element.  Therefore only discrete lines are observed on the spectrum.
Therefore only discrete lines are observed on the spectrum.
(6 marks)
(b) The ionisation energy of a hydrogen atom is 13.6 eV.
(i) State what is meant by the ionisation energy of hydrogen.
The ionisation energy of hydrogen is the energy required to (completely) remove a (ground state/lowest energy level  ) electron from atom/hydrogen
) electron from atom/hydrogen 
(2 marks)
(ii) Express the ionisation energy of hydrogen in joules, giving your answer to an appropriate number of significant figures.
13.6 × 1.6 × 1019 
= 2.18 × 1018 J 
Answer given to 3sf 
(3 marks)
(Total 11 marks)



