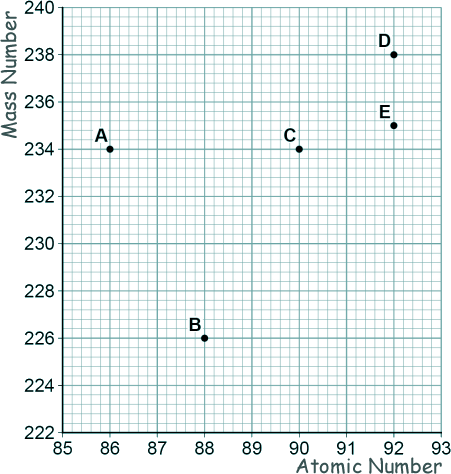
GCSE Questions: Radioactivity Q19. (a) The graph shows a plot for the mass number and the atomic number for the nuclei of five different atoms.
[1 mark]
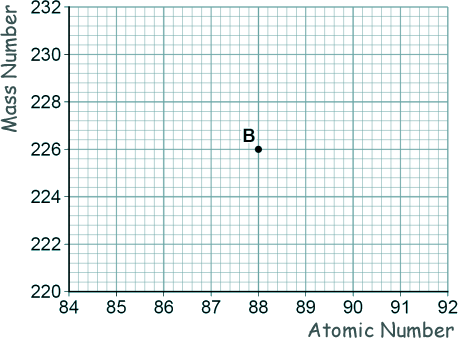
[1 mark] (b) Nucleus B decays by emitting an alpha particle.
Draw an arrow on the diagram to represent the alpha decay. [2 marks] (c) What is meant by the 'random nature of radioactive decay'? [1 mark] (d) A polonium (Po) nucleus decays by emitting an alpha particle and forming a lead (Pb) nucleus. Po → Pb + α The lead (Pb) nucleus then decays by emitting a beta particle and forms a bismuth (Bi) nucleus. Pb → Bi + β The bismuth (Bi) nucleus then decays by emitting a beta particle and forms a polonium (Po) nucleus. Bi → Po + β Explain how these three decays result in a nucleus of the original element, polonium. [3 marks] (Total 8 marks) |
Follow me...
|