Nuclear Fission
Q3. A rod made from uranium-238 ( ) is placed in the core of a nuclear reactor where it absorbs free neutrons. When a nucleus of uranium-238 absorbs a neutron it becomes unstable and decays to neptunium-239 (
) is placed in the core of a nuclear reactor where it absorbs free neutrons. When a nucleus of uranium-238 absorbs a neutron it becomes unstable and decays to neptunium-239 ( ), which in turn decays to plutonium-239 (
), which in turn decays to plutonium-239 ( ).
).
(a) Write down the nuclear equation that represents the decay of neptunium-239 into plutonium-239.
[2 marks]
(b) A sample of the rod is removed from the core and its radiation is monitored from time t = 0 s. The variation of the activity with time is shown below:
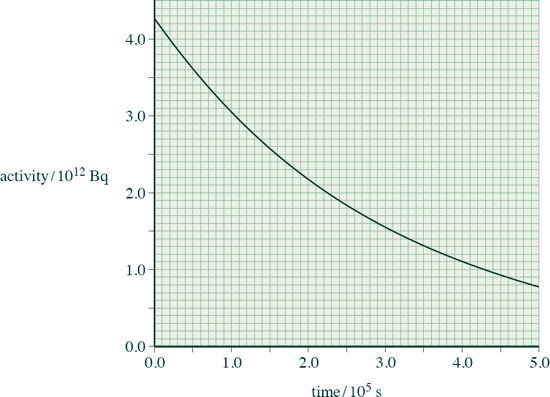
(i) Show that the decay constant of the sample is about 3.4 × 10–6 s–1.
[2 marks]
(ii) Assume that the activity shown in the graph above comes only from the decay of neptunium.
Estimate the number of neptunium nuclei present in the sample at time t = 5.0 × 105 s.
[1 mark]
(c)
(i) A chain reaction is maintained in the core of a thermal nuclear reactor that is operating normally.
Explain what is meant by a chain reaction, naming the materials and particles involved.
[2 marks]
(ii) Explain the purpose of a moderator in a thermal nuclear reactor.
[2 marks]
(iii) Substantial shielding around the core protects nearby workers from the most hazardous radiations. Radiation from the core includes α and β particles, γ rays, X–rays, neutrons and neutrinos.
Explain why the shielding becomes radioactive.
[2 marks]
(Total 11 marks)








