6.4.1
The nuclear atom |
(a) alpha-particle scattering experiment; evidence of a small charged nucleus |
|
(b) simple nuclear model of the atom; protons, neutrons and electrons |
|
(c) relative sizes of atom and nucleus |
(d) proton number; nucleon number; isotopes; notation
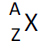
for the representation of nuclei |
| (e) strong nuclear force; short-range nature of the force; attractive to about 3 fm and repulsive below about 0.5 fm
1 fm = 10–15 m |
|
(f) radius of nuclei;
R = r0 A 1/3
where r0 is a constant and
A is the nucleon number |
R = r0 A 1/3 |
|
(g) mean densities of atoms and nuclei |
|
6.4.2
Fundamental particles |
(a) particles and antiparticles; electron–positron, proton-antiproton, neutron-antineutron and neutrino-antineutrino |
|
| (b) particle and its corresponding antiparticle have same mass; electron and positron have opposite charge; proton and antiproton have opposite charge |
(c) classification of hadrons;
proton and neutron as examples of hadrons; all hadrons are subject to both the strong nuclear force and the weak nuclear force |
(d) classification of leptons;
electron and neutrino as examples of leptons;
all leptons are subject to the weak nuclear force but not the strong nuclear force
|
(e) simple quark model of hadrons in terms of:
up (u),
down (d) and
strange (s) quarks and their respective anti-quarks |
(f) quark model of the proton (uud) and the neutron (udd) |
(g) charges of the
up (u),
down (d),
strange (s),
anti-up ( ̅u),
anti-down ( ̅d) and the
anti-strange ( ̅s) quarks as fractions of the elementary charge e |
(h) beta-minus (β-) decay; beta-plus (β+) decay |
(i) β– decay in terms of a quark model; |
(j) β+ decay in terms of a quark model; |
(k) balancing of quark transformation equations in terms of charge |
(l) decay of particles in terms of the quark model. |
6.4.3
Radioactivity |
(a) radioactive decay; spontaneous and random nature of decay |
|
(b) (i) α-particles, β-particles and γ-rays; nature, penetration and range of these radiations |

|
(b) (ii) techniques and procedures used to investigate the absorption of α-particles, β-particles and γ-rays by appropriate materials |
(c) nuclear decay equations for alpha, betaminus and beta-plus decays; balancing nuclear transformation equations |
| (d) activity of a source;
decay constant λ of an isotope;
A = λN |
A = λN |
Learners will also require knowledge of 5.1.4 |
(e) (i) half-life of an isotope;
λ T½ = ln 2 |
λ T½ = ln 2 |
|
(e) (ii) techniques and procedures used to determine the half-life of an isotope such as protactinium |
(f) (i) the equations:
A = A0e-λt
and
N = N0e-λt
where A is the activity and N is the number of undecayed nuclei |
A = A0e-λt
N = N0e-λt |
(f) (ii) simulation of radioactive decay using dice |
(g) graphical methods and spreadsheet modelling of the equation
ΔN/Δt = - λN
for radioactive decay |
ΔN/Δt = - λN |
Using spreadsheets to model the radioactive decay of nuclei.
|
(h) radioactive dating, e.g. carbon-dating. |
|
6.4.4
Nuclear fission and fusion |
(a) Einstein's mass–energy equation;
ΔE = Δmc2 |
ΔE = Δmc2 |

|
(b) energy released (or absorbed) in simple nuclear reactions |
(c) creation and annihilation of particle–antiparticle pairs |
(d) mass defect; binding energy; binding energy per nucleon |
(e) binding energy per nucleon against nucleon number curve; energy changes in reactions |
(f) binding energy of nuclei using
ΔE = Δmc2
and masses of nuclei |
ΔE = Δmc2 |
(g) induced nuclear fission; chain reaction |
|
(h) basic structure of a fission reactor; components – fuel rods, control rods and moderator |
(i) environmental impact of nuclear waste |
Decision making process when building new nuclear power stations. |
(j) nuclear fusion; fusion reactions and temperature |
Learners will also require knowledge of 5.1.4
|
| (k) balancing nuclear transformation equations. |


