|
Activity
|
The
activity of a radioactive sample is the number of emissions of
nuclear radiation from that sample in
one second. It is measured in becquerel
(Bq) |
Alpha
particle
|
An
alpha particle 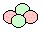 is:
is:
- A particle
of nuclear radiation that is made
up of two protons and two neutrons. It is therefore a helium
nucleus with a nucleon number of 4 and a proton
number of 2. Animated gif: click here
- It has
a mass of 4.0 u (see atomic mass unit)
- Being
massive (on a nuclear scale!) and having a double positive
charge its ionising power is high.
- A nucleus
that has high mass and too many protons to be stable
tends to undergo alpha decay
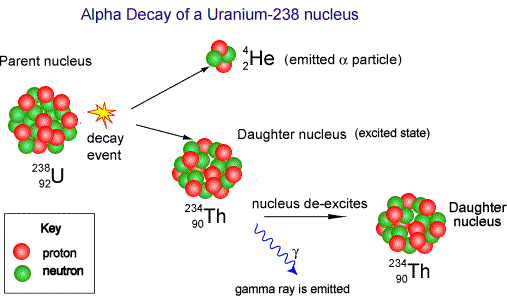
- When
alpha decay occurs a group of two protons and two neutrons
(helium nucleus) comes out of the nucleus. Therefore the proton
number decreases by 2 but the nucleon
number decreases by 4. The resulting daughter
nucleus is of an element 2 positions to the left of the
'parent' in the periodic table.
For an animated gif click
here.
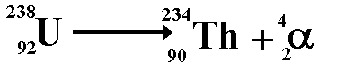
- As its
ionising power is so high it does not penetrate very deeply
into matter before its energy has been used up. Its penetrating
power is therefore very low (absorbed by 10 cm of air,
0.01 mm lead or a sheet of paper).
- The
following symbols are commonly in use:
|
|
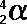
(Greek
letter 'a' - alpha)
|
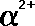
(Greek
letter 'a' - alpha)
|
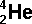
(Chemical
symbol for helium)
|
|
Anion
|
A
negative ion. |
|
Antimatter
|
When
an antimatter particle meets with a matter
particle they annihilate each other! Both particles disappear
and two gamma rays are produced instead.
Antimatter does not get very far through matter before it reaches
its matter counterpart but the gamma rays produced can travel
a long way through matter.
An example
of antimatter is the positron.
|
|
Artificial
Nuclear Transmutation
|
The
human involvement of changing one element
into another by firing neutrons at a nucleus.
On absorbing the neutrons the nucleus changes into a different
isotope. Animated gif: click
here This is often a radioactive
nucleus that then decays to produce
a nucleus of different element. See transmutation.
e.g.
(See transuranic
elements)
|
|
Atom
|
An
atom is the smallest chemically indivisible part of an element.
An atom has a central nucleus composed
of protons and neutrons. The number of
protons in the nucleus of an atom determines
which element the atom is. The elements are listed in the periodic
table. |
|
Click
here to view the image of the atom |
|
Atomic
Mass Unit
|
Atomic
Mass Unit (u): This is a convenient mass unit to use when dealing
with the masses of atoms and nuclear particles. Were the mass
expressed in kilograms the values would be so tiny they would
be difficult for students to deal with.
The unit
is defined by taking the mass of one atom of carbon12
as being 12u. This makes the mass of a proton and a neutron
to be equal to 1u (when measured to the degree of accuracy needed
for GCSE level)
1u
= 1.661 x 10-27 kg
1kg
= 6.020 x 1026 u
(Note -
Some text books use the abbreviation amu)
|
|
Atomic
Number Z
|
(Also
called the proton number, but at GCSE call it the atomic number in exams - using the physicist's term may lose you marks) - The atomic
number of an atom is the number of protons
in the nucleus of the atom. Each element
has its own atomic number. |
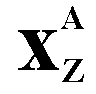 |
|
Background
Radiation
|
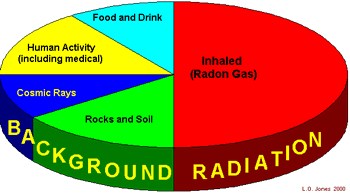 This
comes from five main sources: This
comes from five main sources:
Food and drink
Human activity
Cosmic rays
Rocks and Soil Radon |
|
Background
Rate
|
The
activity from background radiation. If
a Geiger-counter is switched on in a room
it will register ionising radiation. This
will vary according to location (see background
radiation).
Before an
experiment with a radioactive source is carried out the background
rate must be established. This is done by switching on the Geiger-counter
when the source is not present and recording the average
activity for twenty minutes or so. This value
must then be deducted from any readings taken with the source
present so that the true activity of the sample can be found.
|
|
Becquerel
|
This
is the unit of activity of a radioisotope.
It is the number of spontaneous nuclear transformations in one
second.
The abbreviation
is Bq. (The old
unit is the curie)
|
|
Beta
Particle
|
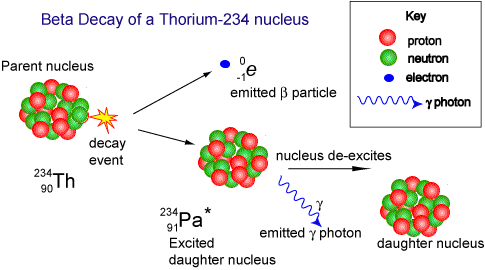
A
beta particle  is:
is:
- a particle
of nuclear radiation that is a fast
electron. Animated gif: click
here
- It has
a mass of 0.000055 u (see atomic mass unit)
- Being
of tiny mass (even on a nuclear scale) and having only a single
negative charge its ionising power
is low compared to the alpha particle.
- As its
ionising power is low it can penetrate quite deeply into matter
before its energy has been used up. Its penetrating
power is therefore moderate (absorbed by 1m air, 0.1 mm
lead or 3mm aluminium sheet).
- When
a nucleus has too many neutrons, it
tends to beta decay.
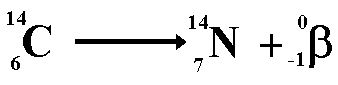
- The
following symbols are commonly in use:
|
|
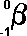
(Greek
letter 'b' - beta)
|
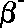
(Greek
letter 'b' - beta)
|
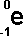
('e' stands
for electron)
|
|
|
The
mass of the nucleus is less than the total mass its nucleons would
have if they were separated. The mass defect
is the difference in mass between the total mass of the separated
nucleons and the mass of the nucleus. The mass defect is equivalent
to the energy released when the nucleons are bound.
Binding
energy is the energy that must be supplied to the nucleus in
order to separate it into its nucleons.
The binding
energy is the energy equivalent of the mass defect.
|
|
Carbon
Dating
|
Methods
of finding out the age of artefacts that contain materials that
were once alive. When it dies a living organism takes in no more
carbon from the atmosphere and the percentage of C-14 will decrease.
The ratio of C-14 to total carbon is found and from this the age
can be calculated. |
|
Cation
|
A
positive ion. |
|
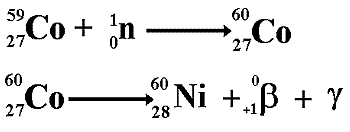
Cobalt
|
Chemical
symbol (Co),
Cobalt was
discovered in 1735 by Georg Brandt.
Description:
a silver-white, lustrous, hard metal that can be magnetised.
Naturally occurring (with other metals) as the ores cobaltite
and smaltite Cobalt alloys are used in very hard cutting tools,
high-strength permanent magnets, and jet engines.
A radioactive
form of cobalt, cobalt-60, is prepared by exposing naturally
occurring cobalt-59 to the radiations of an atomic pile.
The nucleus
absorbs a neutron and becomes cobalt-60. This is a gamma and
beta emitter. The beta rays are of low
energy (said to be 'soft') and are easily absorbed by a metal
coating on top of a cobalt source. The cobalt is then effectively
only giving gamma rays to the surroundings.
It has a
half life of 5.3 years and decays to
produce a daughter nuclide of nickel-60
(Stable)
It is used:
- in
industry in the inspection of materials to reveal internal
structure, flaws, or foreign objects or as a radioactive
tracer and
- in medical
science in cancer therapy.
|
|
Collimator
|
A
device that produces a parallel beam of radiation. This is either
done by refraction (as with light), bending the rays until they
are parallel using a system of lenses or (as with gamma
rays) by absorbing (using a lead grid system) all of those rays
that are not travelling parallel to one another. |
|
Compound
|
Two
or more different atoms that are chemically
bonded together form molecules of a compound. The compound often
has very different properties from the elements
it is made up of. |
|
Cosmic
Rays
|
High-energy
particles of ionising radiation from space.
(See Appendix
B)
|
|
Curie
|
The
Curie (Ci) is an old unit of activity. One curie is a very large
amount of radioactivity. It is more common to find activities
given in millicurie or microcurie
1 Ci = 37
GBq = 37,000,000,000 Bq
|
|
Daughter
Nucleus
|
The
name given to a decay product of the
parent nucleus. |
Decay
product
|
A
nuclide or radionuclide produced by radioactive
decay. It may be formed directly from a radionuclide or as
a result of a series of successive decays through several radionuclides. |
|
DNA
|
Deoxyribonucleic
Acid - double helix molecule that forms the genetic material of
all living cells. It controls the structure and function of cells
and is the material of inheritance; therefore damage to the coding
can cause mutation.
Ionising
radiation can cause damage to the structure of DNA so that
on division the new cell is altered (mutated). Repair to damage
to DNA is carried out by 'repair enzymes'. If mutants that lack
such a facility are more susceptible to radiation damage and
to express the damage so produced. Nuclear
radiation therefore can have harmful effects on living
matter, resulting in mutations and cancer (tumours). Very high
dose can cause cell death.
|
|
Electromagnetic
radiation
|
All
electromagnetic radiation is pure energy (zero mass) and part
of the electromagnetic spectrum. Light
energy is the most familiar part of this.
All electromagnetic
radiation travels at a speed of 3.0 x 108 m/s in
a vacuum.
|
|
Electromagnetic
Spectrum
|
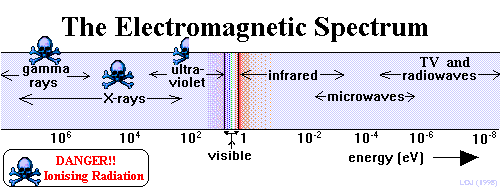 |
|
This
is a continuous band of electromagnetic radiation arranged in
the order of decreasing photon energy, decreasing frequency or
increasing wavelength. Each part is named according to its origin
and frequency/wavelength range. Light energy is the most familiar
part of the spectrum and it is often referred to as the 'family
of light'. Some parts of the e.m. spectrum can be directly detected
by humans, others cannot.
See the
table below:  indicates that the rays are harmful because they are of high
enough energy to be ionising radiation.
indicates that the rays are harmful because they are of high
enough energy to be ionising radiation.
|
|
Name
|
Typical
wavelength (m)
|
Sources
|
Detectors
|
|
Gamma
Rays
g-rays

|
10-12
|
Nucleus of
radioactive atom
|
Geiger-Muller
Tube
|
|
X-rays

|
10-10
|
X-ray tube
|
Photographic
film
|
|
Ultraviolet
(UV)

|
10-8
|
Very hot
objects, the Sun, sparks, mercury lamps
|
Photographic
film, causes a sun tan, makes fluorescent substances glow
|
|
visible
|
10-7
|
Hot objects,
the Sun, fluorescent substances, lasers
|
Human eye,
photographic film, LDR
|
|
infra-red
|
10-5
|
Warm or hot
objects, the Sun
|
Skin, blackened
thermometer, thermistor
|
|
microwave
|
10-2
|
Magnetron
- found in microwave ovens, mobile phone transmitters, satellite
communications
|
Aerial with
a microwave receiver set
|
|
TV and
radio
|
10+2
|
Radio transmitters,
radar transmitters
|
Aerial with
a radio/TV receiver set
|
|
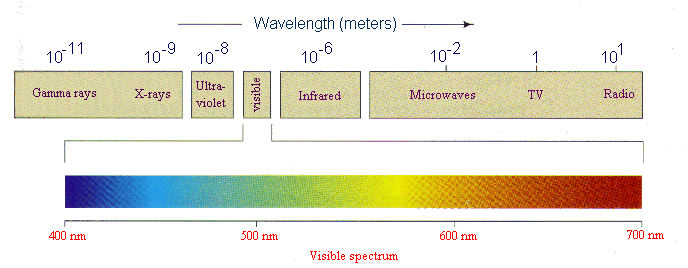
Click
on graphic to enlarge
|
|
Electron
|
 Sub-atomic particle that orbits the nucleus
of the atom. It has a mass of 0.00055u (atomic
mass units) and unit negative charge (-1).
Sub-atomic particle that orbits the nucleus
of the atom. It has a mass of 0.00055u (atomic
mass units) and unit negative charge (-1). |
|
Electron
volt
|
Energy
can be calculated in terms of electron volts (eV)
The eV is
a very small unit of energy. It is used a lot in nuclear calculations. From GCSE
electricity we know that
|
|
E
(joule)
|
=
|
Q
(coulomb)
|
V
(volt)
|
|
So,
a joule could be thought of as a 'coulomb volt'
Similarly,
if we don't use a full coulomb of charge, but only the charge
on an electron 'e'
|
|
E
(electron
volt)
|
=
|
Q
(change
on one electron)
|
V
(volt)
|
|
The
charge on an electron is only 1.6 x 10-19 C
Therefore
the eV is only 1.6 x 10-19 J
=
0.000 000 000 000 000 000 19 J (very tiny!)
|
|
Element
|
An
element is a substance that contains only one type of atom
(all of its atoms have the same proton number).
It cannot be chemically broken down into any simpler substance.
Elements are listed in the periodic table. |
|
Fundamental
Forces of Nature
|
|
Name |
Domain |
Exchange
Particle |
Relative
strength
|
|
I
|
Strong
Nuclear Force |
Within
the nucleus - acts between nucleons over a very short range |
Pions
(mesons) |
1
|
|
II
|
Electromagnetic
Force |
Between
charged particles - binds atoms and molecules together |
photons |
10-2
|
|
III
|
Weak
Nuclear Force |
Within
the nucleus - governs radioactive beta decay |
involves
leptons w bosons and z-particles |
10-6
|
|
IV
|
Gravitational
Force |
Acts
between all masses - very important for large masses in space
such as planets and stars |
gravitons
(not yet detected!) |
10-38
|
|
Gamma
Camera
|
An
imaging device that formulates pictures based upon spatial distribution
of gamma ray intensities. For more details and diagrams see medical
applications section of human activities |
|
Gamma
Ray
|
The
gamma ray  is: is:
- As its
ionising power is so low it penetrates very deeply into matter
before its energy has been used up. Its penetrating
power is therefore very high (about 99.9% is absorbed
by 1 km of air or 10 cm lead). Very few of the gamma rays
emitted from the Sun reach the Earth's surface because the
atmosphere is thick enough to absorb virtually all of them.
- For
gamma ray emission to occur the nucleus must be in an excited
state after emitting an alpha, beta or positron particle.
Sometimes it stays like that for quite a while before the
gamma ray is emitted (see metastable state),
sometimes it is instantaneous. When gamma emission occurs
there is no emission of matter particles
therefore the nucleon number and
the proton number remain the same.
The remaining nucleus is of the same
isotope but at a lower energy state.
-
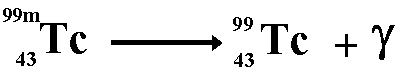
- The
following symbol is commonly in use:
|
|
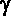 (Greek
letter 'g' - gamma) (Greek
letter 'g' - gamma)
|
|
Geiger-Müller
Tube
|
Often
called a Geiger-counter the GM Tube is used to count the activity
of a radioactive sample. It is a special type of ionisation chamber
that can be used to detect alpha, beta
or gamma radiation. The output of the tube
is a voltage that can either be connected to a scaler that gives
an accumulated total of counts or a rate meter that gives an output
in becquerel. Sometimes the output is
a speaker that gives a loud 'click' for each particle it detects.
The output can also be connected via an interface to a computer. |
|
Gray
|
The
gray (Gy) is the unit of absorbed dose. |
|
Half
Life
|
The
half-life of a radioisotope is the
time taken for half of the atoms in a sample of that radionuclide
to decay (emit nuclear radiation). It
can also be expressed as the time taken for the activity
of the sample to halve.
It has the
following accepted symbols:
|
|
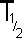
|
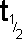
|
|
Ion
|
An
ion is an atom that is being orbited by a
different number of electrons than the
number of protons in its nucleus.
If it has too many electrons to be neutral it will have a negative
charge and be called an anion. If it has
too few electrons to be neutral it will have a positive charge
and be called a cation. |
|
Ionization
|
The
process by which a neutral atom gains or loses
an electron thereby becoming an ion. |
|
Ionizing
power
|
The
ionizing power of ionising radiation measures
how many ions are formed in a given area when
the radiation passes through it. Alpha particles
have a double charge and are very massive compared to beta and
gamma they are therefore very strongly ionizing, interacting with
many electrons in the orbitals of atoms before they lose their
energy. Beta particles with their much smaller
mass and single charge have less ionising power. The least ionizing
radiation is composed of gamma photons because
it is neutral and has no mass.
Ionizing
power is often quoted as ion pairs per centimetre of air.
|
|
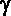
(~10/cm)
low power
|
<
|
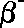
(~103/cm)
medium
power
|
<
|
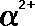
(~105/cm)
high power
|
|
It
indicates how densely packed incidents of ionisation will be.
In human tissue ionisation occurring in a limited location means
concentrated tissue damage and will increase the probability of
cell death or mutations due to DNA damage.
NB
- the ionising power comparison is for beams of comparable
intensity.
|
|
Ionizing
radiation
|
Radiation
of high enough energy to cause ionization.
The energy of a photon of electromagnetic radiation
or nuclear radiation is given to an
orbital electron. If it is sufficiently
high enough to promote the electron out of the influence of its
nucleus, it is ionizing radiation and an
ion will be formed. |
|
Isotope
|
An
element can exist in several different
isotopes. Each isotope of an element has the same proton number
but a different number of neutrons, making the nucleon
number different. All isotopes of an element have the same
chemical properties (the way they react with other substances)
but different physical properties (boiling point, melting point,
density etc.). Some isotopes are more common than others but there
is no such thing as a 'normal' isotope. The nucleus
of some isotopes is unstable, such isotopes are termed radioisotopes. |
|
LINAC
- linear accelerator
|
A
linear accelerator produces X-rays of such
high energy that they are in the same energy range as gamma
rays. They can then be used fro radiotherapy. The advantage
of the LINAC over a fixed radioactive source is that it can be
switched off when not in use, whereas the cobalt 60 source has
to be sealed in lead and treated with care when not in use. They
can also produce rays just of a required energy and intensity.
However
they are very expensive to buy and not all hospitals can afford
one.
|
| Mass
defect |
The
mass of the nucleus is less than the total mass its nucleons would
have if they were separated. The mass defect is the difference
in mass between the total mass of the separated nucleons and the
mass of the nucleus. The mass defect is equivalent to the energy
released when the nucleons are bound.
Binding
energy is the energy that must be supplied to the nucleus in
order to separate it into its nucleons.
The binding
energy is the energy equivalent of the mass defect.
|
|
Mass
Number A
|
Mass
number is the average number of nucleons found in a large sample
of an element. Naturally occurring chlorine exists in the form
of two isotopes Cl37 (25%) and Cl35 (75%).
Therefore the 'average' mass of a chlorine atom is 35.5. Chemists
use mass number because they deal with large quantities of atoms.
Physicists prefer the term nucleon number
and deal with individual isotopes rather
than the naturally occurring mixture of them, but the GCSE syllabuses like you to use the term mass number - so do that at that level! |
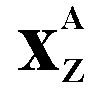 |
|
Matter
|
Matter
is anything which occupies space. There are three states of matter:
solid, liquid and gas. It is made up of atoms. |
|
Metastable
State
|
Sometimes,
after its emission of an alpha, beta
or positron particle, the nucleus
is still in an excited state, called a metastable state. In order
to get to a lower energy state it emits a quantum
of energy in the form of a gamma ray.
No matter
is emitted from the nucleus therefore the nucleon
number and the proton number remain
the same. Before and after emission of the gamma ray they are
the same isotope of the element
but they are different nuclide because
the term nuclide incorporates nuclear energy states as well
basic structure.
|
|
Neutron
|
 Nuclear
particle that has zero charge and a mass of 1u (atomic
mass unit) Nuclear
particle that has zero charge and a mass of 1u (atomic
mass unit) |
|
Nuclear
radiation
|
Nuclear
radiation emanates from the nucleus of
an atom. It is ionising
radiation. There are five types of nuclear radiation, three
are made of matter: alpha,
beta and the neutron. Gamma is electromagnetic radiation and the
fifth is an antimatter particle: the
positron. |
|
Alpha |
α |
2 protons and 2 neutrons
(a helium nucleus)
|
+2 |
4 |
Beta |
β+ |
|
-1 |
Negligible |
Positron |
β- |
|
+1 |
Negligible |
Gamma |
γ |
photons of
electromagnetic radiation
|
0 |
0 |
Neutron |
n |
|
0 |
1 |
|
|
Nucleon
|
The
name given to a particle within the nucleus (a proton
OR an neutron) |
|
Nucleon
Number A
|
(See
mass number) - The nucleon number of an atom is the number of
neutrons in the nucleus
of an atom. Each element can have several
different nucleon numbers. Each one corresponds to a different
isotope of the element. |
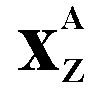 |
|
Nuclei
|
Plural
form of nucleus (Latin nouns ending in 'us' usually have plural
forms that end in 'i') |
|
Nucleus
|
The
nucleus of an atom contains all of its protons
and neutrons. It is a tiny dense centre
to an atom that is orbited by its electrons.
- Nuclear
sizes are expressed in femtometres (10-15 m),
where as atomic sizes are expressed in 10-10 m.
This means that an atom's diameter is about 100.000 times
bigger than its nucleus!
If you made
a model of an atom (to scale) and had the diameter of the nucleus
to be 1mm (a peppercorn) the diameter of the atom itself would
have to be 100m!
- Nuclear
mass is virtually the same as the atomic mass because
the electrons have such tiny mass compared to the mass of
electrons and protons.
|
|
Nuclide
|
An
atom defined by its proton number, nucleon
number and energy state. Thus it is not
only clearly identified as a particular isotope
of that atom but also its energy state is clearly defined. |
|
|
A particle
accelerator is a device used to project charged particles at
high speed into matter. Electric and magnetic fields are used
to provide the forces to accelerate and control these charged
particles at speeds approaching the speed of light. When these
charged particles strike the nucleus
of an atom, they alter its stability and new particles may be
produced.Various devices used to detect subatomic particles
are the Geiger counter, ionization chamber, bubble chamber,
cloud chamber, spark chamber, scintillation counter, and photographic
film.
|
|
Penetrating
Power
|
The
penetrating power of nuclear radiation
depends upon the ionising power of the
radiation. The radiation continues to penetrate matter until it
has lost all of its energy. The more localised the ionisation
the less penetrating power it will possess.
Alpha
particles are the least penetrating as they are the most
densely ionising; beta particles are more
penetrating than alpha because they are less ionising and gamma
have the most penetrating power.
Click here to view this
as an animation
|
|
Periodic
Table
|
The
Periodic table is a chart used primarily by chemists. It summarises
the elements by arranging them in the order
of their atomic number in such a way that similarities and trends
can be appreciated.
Each row
(or PERIOD of the table) exhibits a range of properties
that can be interpreted by how the electron orbitals of the
atom are filled. Each column or GROUP of elements exhibits
similar properties (like a family show similar characteristics).
|
|
PET
|
Positron
emission tomography. (More details) |
|
Photoelectric
Effect
|
The
emission of electrons from matter by photons
of electromagnetic radiation. 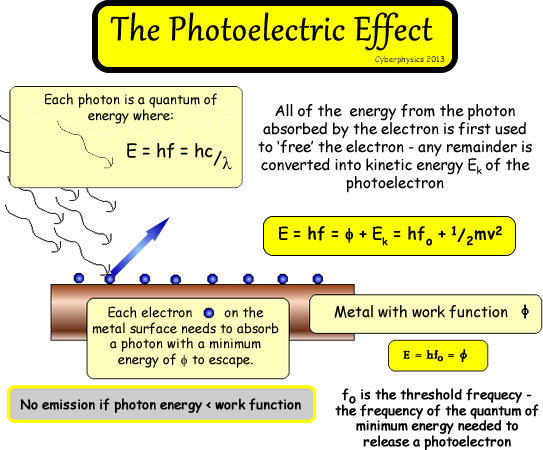 |
|
Photomultiplier
|
A
photomultiplier is a device in which incident photons
create measurable electrical pulses. The device is based on the
photoelectric effect. It uses large
electric fields to accelerate electrons and, through a cascade
sequence, amplify the signal. When it is large enough it can be
sent to electronic circuitry for analysis and display. |
|
Photon
|
A
photon is a name given to a quantum of
electromagnetic energy. It is usually used to refer to photons
of visible light if the part of the electromagnetic
spectrum is not otherwise specified. |
|
Positron
|
A
positron  is an antimatterbeta
particle:
is an antimatterbeta
particle:
- When
it meets with an electron it annihilates it! Both particles
disappear and two gamma rays are produced
instead. It does not get very far through matter before it
reaches another electron therefore its ionising
power is great.
- As its
ionising power is great it cannot penetrate quite deeply into
matter before it is annihilated. Its penetrating
power is therefore very low indeed but it produces gamma
rays and these have great penetration power.
- When
a nucleus has too many protons,
it tends to positron decay.
- When
positron decay occurs a proton within
the nucleus emits the particle and
changes into a neutron. Animated gif:
click here .Therefore the proton
number decreases but the nucleon
number stays the same (only now you have one more neutron
and one less proton!). The resulting daughter
nucleus is of an element 1 position to the left of the
'parent' in the periodic table.
For an animated gif click
here.
-
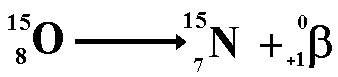
- The
following symbols are commonly in use:
|
|
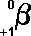
(Greek
letter 'b' - beta)
|
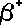
(Greek
letter 'b' - beta)
|
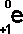
('e' stands
for electron)
|
|
Proton
|
 Nuclear
particle that has unit positive charge (+1) and a mass of 1u (atomic
mass unit). The number of protons (proton
number) in a nucleus determines which element the atom
belongs to. Nuclear
particle that has unit positive charge (+1) and a mass of 1u (atomic
mass unit). The number of protons (proton
number) in a nucleus determines which element the atom
belongs to. |
|
Proton
Number Z
|
(Also
called the atomic number) - The proton
number of an atom is the number of protons
in the nucleus of an atom.
Each element has its own proton number. |
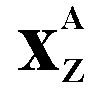 |
|
Quantum
of Energy
|
A
quantum of energy is a little packet of energy. The quantum theory
developed at the beginning of the last century tells us that energy
changes in atoms happens when these little packets of energy are
taken in or given out. The value of the quantum of energy can
be calculated using the equation:
E =
hf
where:
E
is the energy in joules
h
is the Planck constant
and
f is the frequency in hertz
|
|
Rad
|
The
old unit for absorbed dose was the 'rad'
1rad =
0.01 J/kg = 0.01Gy
|
|
Radiation
Dose
|
- Absorbed
dose (D) is measured in gray (Gy). This
is a measure of how much energy is absorbed by unit mass from
the ionising radiation it is exposed
to.
1Gy = 1 J/kg
The old
unit for absorbed dose was the 'rad'
1rad =
0.01 J/kg = 0.01Gy
- Dose
equivalent (H) is measured in sievert (Sv).
It takes into consideration not only the intensity of
the radiation source and the time of exposure but also
the different ionising powers
(and therefore potential hazard) to the recipient.
The old
unit for dose equivalent is the rem
1 rem
= 0.01 Sv
|
|
Radioactive
|
A
word used to describe substances that contain atoms that emit
nuclear radiation. How radioactive a
substance is depends on its activity. |
|
Radioactive
Decay
|
It
is the process of spontaneous transformation of a radionuclide
by the emission of nuclear radiation.
Radioactive
nuclear decay occurs whenever a nucleus is in an energy-state
that is not the lowest possible for its nucleon
number. This state may occur naturally (which essentially
means that it was created in that state when formed within a
star) or by artificial means (neutron or photon irradiation).
 The
emission of the nuclear radiation
is a purely random event. It cannot be predicted exactly when
an atom will decay, only that a certain number will decay
in a given time. The mathematics of probability is used for
this requires a large number of atoms to be considered. (See
half life and radioactive
decay series). The
emission of the nuclear radiation
is a purely random event. It cannot be predicted exactly when
an atom will decay, only that a certain number will decay
in a given time. The mathematics of probability is used for
this requires a large number of atoms to be considered. (See
half life and radioactive
decay series).
 The
nucleus remaining is called the decay
product or daughter nucleus. The
nucleus remaining is called the decay
product or daughter nucleus.
 The
rate of decay depends on the number of undecayed nuclei present,
so with each decay event there is a decrease in the activity
of a radioactive sample. The
rate of decay depends on the number of undecayed nuclei present,
so with each decay event there is a decrease in the activity
of a radioactive sample.
|
|
Radioactive
Decay Series
|
When
ever a decay occurs the nucleon
number either stays the same (gamma or
beta emission) or reduces by four (alpha
emission). Therefore if you divide the nucleon number by four
you will either have no remainder or 1, 2 or 3. Mathematically
we could say that the nucleon number could be described by 4n,
4n+1, 4n+2 or 4n+3.
See here.
|
|
Radioactive
Label
|
See
radioactive tracer. |
|
Radioactive
Tracer
|
A
small amount of radioisotope is made
to replace a non-radioactive isotope of an element
in a compound. The path of that radioisotope
or one of its daughter nuclei (decomposition
product) is then monitored by detection of emitted nuclear
radiation. It is also called a radioactive
label. |
|
Radioactivity
|
The
property of a radionuclide to spontaneously
emit ionising radiation. It arises from
the breakdown of an unstable nucleus |
|
Radioisotope
|
A
radioisotope is an isotope that has an
unstable nucleus. The nucleus emits a nuclear
radiation to attain stability by a process called radioactive
decay. |
|
Radionuclide
|
An
atom of a radioisotope. A radioactive
atom defined by its proton number, nucleon
number and energy state. Thus it is not only clearly identified
as a particular radioisotope of that
atom but also its energy state is clearly defined giving an indication
of whether it will emit a gamma ray to attain
a more stable lower energy state. |
|
Radiosensitive
|
Some
biological cells divide more rapidly than others. these are the
ones that are more likely to suffer damage from ionising
radiation. They do not have sufficient time to 'repair damage'
before they are required to divide again. Cancer cells fall into
this category. |
|
Radiotherapy
|
The
destruction of malignant tumours with a high dose of gamma
radiation that will result in cell death
See radiation
therapy for more details
|
|
Rate
of Decay
|
This
is the number of particles of nuclear radiation
emitted in one second. It is measured in becquerel. |
|
Rem
|
The
old unit for dose equivalent.
1 rem
= 0.01 Sv
|
|
Sievert
|
The
unit for the dose equivalent of ionising
radiation received by a person.
The abbreviation
is Sv. A dose of
1 Sv is very high. The maximum annual permitted dose level (PDL)
for the average person (i.e. one not working with ionising radiation)
is just 5mSv - the average dose is about 2mSv.
|
| Specific Charge |
The specific charge of a nucleus is the total charge of the nucleons (in coulombs) divided by the total mass of the nucleons (in kilograms). Specific charge is measured in C-1kg |
|
Strong
Nuclear Force
|
This
is the strongest of the four fundamental
forces of nature. It only has a very short range - to the
order of femtometres (10-15m). It is the force that
holds the nucleus together. |
|
Tc
99m
|
Technetium
99m is a very useful radioisotope.
It is the most commonly used one in hospitals because it is ideal
to use with a gamma camera and only
emits gamma rays. (See here) |
|
Transmutation
|
Transmutation
is the process by which the nucleus of
a radioactive atom undergoes decay
into an atom with a different number of protons, until such time
as a stable nucleus is produced. See artificial
transmutation. (See the simulation) |
|
Transuranic
Elements
|
These
are elements with proton
numbers greater than 92. The transuranic elements are not
naturally occurring, they are artificially created.
They were
made by firing neutrons at the nuclei
of heavy atoms such as uranium. These are
then neutron rich and decay by beta
emission to produce atoms with bigger proton numbers. This
is called artificial transmutation.
The naturally occurring isotopes decay
by alpha emission thereby reducing the
proton number. It is only since the dawn of the nuclear age
that elements with proton numbers bigger than 92 have existed
on our planet.
|
|
X-ray
|
The
X-ray is:
- a photon
of high energy, short wavelength electomagnetic
radiation that is not made up matter at all.. it is pure energy.
- the
result of an atomic electron transition for a very heavy element.
- It is
produced in an X-ray tube. High energy electrons
are fired at a metal (usually tungsten) target within an evacuated
tube. The interaction of these high energy electrons with
the electrons orbiting the metal atom produces X-rays - they
are NOT nuclear radiation (cf gamma ray
production). Some very powerful X-ray machines can produce
X-rays that are of higher energy than most gamma rays. (see
LINAC)
- Having
no mass and no charge its ionising power
is very low.
- As its
ionising power is so low it penetrates
very deeply into matter before its energy has been used up.
Its penetrating power is therefore
very high (about 99.9% is absorbed by 100 m of air or 1cm
lead).
|
|
Zone
of nuclear stability
|
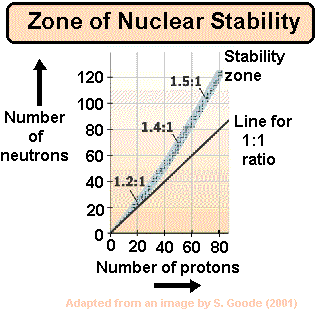
The ratio
of neutrons to protons
in the nucleus increases from 1:1 as
the nucleon number of stable isotopes
increases. Those lying outside the zone are generally radioactive
and those within it generally stable.
|


